Students can Download Samacheer Kalvi 10th Science Model Question Paper 2 English Medium Pdf, Samacheer Kalvi 10th Science Model Question Papers helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamil Nadu Samacheer Kalvi 10th Science Model Question Paper 2 English Medium
General Instructions:
- The question paper comprises of four parts
- You are to attempt all the questions in each part. An internal choice of questions is provided wherever applicable.
- All questions of Part I, II, III and IV are to be attempted separately.
- Question numbers 1 to 12 in Part I are Multiple Choice Questions of one mark each.
These are to be answered by writing the correct answer along with the corresponding option code. - Question numbers 13 to 22 in Part II are of two marks each. Any one question should be answered compulsorily.
- Question numbers 23 to 32 in Part III are of four marks each. Any one question should be answered compulsorily.
- Question numbers 33 to 35 in Part IV are of seven marks each. Draw diagrams wherever necessary.
Time: 3 Hours
Maximum Marks: 75
Part – I
(i) Answer all the questions. [12 × 1 = 12]
(ii) Choose the most suitable answer and write the code with the corresponding answer.
Question 1.
Inertia of a body depends on ______.
(a) weight of the object
(b) acceleration due to gravity of the planet
(c) mass of the object
(d) Both a & b
Answer:
(c) mass of the object
Question 2.
Magnification of a convex lens is _______.
(a) Positive
(b) Negative
(c) either positive or negative
(d) zero
Answer:
(b) Negative
![]()
Question 3.
When a sound wave travels through air, the air particles _______.
(a) vibrate along the direction of the wave motion
(b) vibrate but not in any fixed direction
(c) vibrate perpendicular to the direction of the wave motion
(d) do not vibrate
Answer:
(a) vibrate along the direction of the wave motion
Question 4.
The number of neutrons is 8O16 is _______.
(a) 8
(b) 16
(c) 32
(d) 24
Answer:
(a) 8
Question 5.
Which of the following is a triatomic molecule?
(a) Glucose
(b) Helium
(c) Carbon-di-oxide
(d) Hydrogen
Answer:
(c) Carbon-di-oxide
Question 6.
Photolysis is a decomposition reaction caused by ______.
(a) heat
(b) electricity
(c) light
(d) mechanical energy
Answer:
(c) light
Question 7.
Water which is absorbed by roots is transported to aerial parts of the plant through ________.
(a) phloem
(b) epidermis
(c) cortex
(d) xylem
Answer:
(d) xylem
![]()
Question 8.
World ‘No Tobacco Day’ is observed on _______.
(a) May 31st
(b) April 22nd
(c) June 6th
(d) October 2nd
Answer:
(a) May 31st
Question 9.
Oxygen is produced at what point during photosynthesis?
(a) when ATP is converted to ADP
(b) when CO2 is fixed
(c) when H2O is splitted
(d) All of these
Answer:
(b) when CO2 is fixed
Question 10.
The phenomenon by which carbohydrates are oxidised to release ethyl alcohol is
(a) Glycolysis
(b) Kreb’s cycle
(c) Photosynthesis
(d) Fermentation
Answer:
(d) Fermentation
Question 11.
Biogenetic law states that ______.
(a) Ontogeny and phylogeny go together
(b) Ontogeny recapitulates phylogeny
(c) Phylogeny recapitulates ontogeny
(d) There is no relationship between phylogeny and ontogeny
Answer:
(b) Ontogeny recapitulates phylogeny
![]()
Question 12.
Where you will create category of blocks?
(a) Block palette
(b) Block menu
(c) Script area
(d) Sprite
Answer:
(b) Block menu
Part – II
Answer any seven questions. (Q.No: 22 is compulsory) [7 × 2 = 14]
Question 13.
State Boyle’s law.
Answer:
When the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure. P ∝ \(\frac{1}{V}\)
Question 14.
Differentiate mass and weight.
Answer:
| Mass | Weight |
| 1. The quantity of matter contained in the body | 1. The gravitation force exerted on it due to the Earth’s gravity alone. |
| 2. Scalar quantity | 2. Vector quantity |
| 3. Unit: kg | 3. Unit: N |
| 4. Constant at all the places | 4. Variable with respect to gravity. |
Question 15.
Why are traffic signals red in colour?
Answer:
- Red light has the highest wavelength.
- It is scattered by atmospheric particles.
- So red light is able to travel the longest distance through fog, rain etc.
![]()
Question 16.
Define volume percentage.
Answer:
Volume percentage is defined as the percentage by volume of solute (in ml) present in the given volume of the solution.
![]()
Question 17.
Give any two examples for heterodiatomic molecules.
Answer:
Heterodiatomic molecules -E.g: HCl, NaCl.
Question 18.
Match the following:

Answer:
(a) (ii)
(b) (iv)
(c) (iii)
(d) (i)
Question 19.
Write the differences between endocrine and exocrine gland.
Answer:
| Endocrine | Exocrine |
| 1. Secretion of endocrines are hormones | 1. Secretion of exocrines are enzymes |
| 2. Endocrine glands do not have specific duct hence ductless gland. | 2. Exocrine glands have specific duct to carry their secretions. |
| 3. Eg. Pituitary gland | 3. Eg. Salivary gland |
Question 20.
Identify the parts A,B,C and D.
Answer:
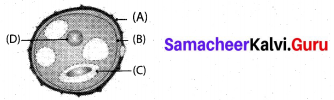
A – Exine, B – Intine, C – Generative cell, D – Vegetative nucleus
![]()
Question 21.
Define Cancer.
Answer:
Cancer is an abnormal and uncontrolled division of cells that invade and destroy surrounding tissue forming a tumor or neoplasm: It is a heterogenous group of cells that do not respond to the normal cell division.
Question 22.
The potential difference between two conductor is 110 V. How much work moving 5C charge from one conductor to the other?
Answer:
Given, V = 110 V , Charge q = 5C
V = \(\frac{W}{q}\)
W = V × q
W = 110 × 5 = 550 J
∴ Work done W = 550 J
Part – III
Answer any seven questions (Q.No: 32 is compulsory) [7 × 4 = 28]
Question 23.
Describe the rocket propulsion.
Answer:
- Propulsion of rockets is based on the law of conservation of linear momentum as well as Newton’s III law of motion.
- Rockets are filled with a fuel (either liquid or solid) in the propellant tank. When the rocket is fired, this fuel is burnt and a hot gas is ejected with a high speed from the nozzle of the rocket, producing a huge momentum.
- To balance this momentum, an equal and opposite reaction force is produced in the combustion chamber, which makes the rocket project forward.
- While in motion, the mass of the rocket gradually decreases, until the fuel is completely burnt out.
- Since, there is no net external force acting on it, the linear momentum of the system is conserved.
- The mass of the rocket decreases with altitude, which results in the gradual increase in velocity of the rocket.
- At one stage, it reaches a velocity, which is sufficient to just escape from the gravitational pull of the Earth. This velocity is called escape velocity.
Question 24.
Explain the types of Scattering.
Answer:
Types of Scattering.
When a beam of light, interacts with a constituent particle of the medium, it undergoes many kinds of scattering.
Based on initial and final energy of the light beam, scattering can be classified as,
- Elastic scattering
- Inelastic scattering
1. Elastic scattering:
If the energy of the incident beam of light and the scattered beam of light are same, then it is called as ‘elastic scattering’.
2. Inelastic scattering:
If the energy of the incident beam of light and the scattered beam of light are not same, then it is called as ‘inelastic scattering’.
The nature and size of the scattered results in different types of scattering. They are
- Raylight scattering
- Mie scattering
- Tyndall scattering
- Raman scattering
![]()
Question 25.
(a) Calculate the current and the resistance of a 100 W, 200 V electric bulb in an electric circuit.
Answer:
Given, P = 100 W, V = 200 V
Power P = VI
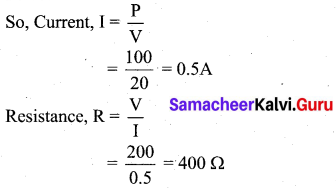
(b) What is the role of the earth wire in domestic circuits?
Answer:
- The earth wire provides a low resistance path to the electric current.
- The earth wire sends the current from the body of the appliance to the Earth, whenever a live wire accidentally touches the body of the metallic electric appliance.
- Thus, the earth wire serves as a protective conductor, which saves us from electric shocks.
Question 26.
Explain the mechanism of cleansing action of soap.
Answer:
A soap molecule contains two chemically distinct parts that interact differently with water. It has one polar end, which is a short head with a carboxylate group (-COONa) and one nonpolar end having the long tail made of the hydrocarbon chain.
The polar end is hydrophilic (Water loving) in nature and this end is attracted towards water. The non-polar end is hydrophobic (Water hating) in nature and it is attracted towards dirt or oil on the cloth, but not attracted towards water. Thus, the hydrophobic part of the soap molecule traps the dirt and the hydrophilic part makes the entire molecule soluble in water.
When a soap or detergent is dissolved in water, the molecules join together as clusters called ‘micelles’. Their long hydrocarbon chains attach themselves to the oil and dirt. The dirt is thus surrounded by the non-polar end of the soap molecules. The charged carboxylate end of the soap molecules makes the micelles soluble in water. Thus, the dirt is washed away with the soap.
Question 27.
(a) Explain the various types of binary solutions based on the physical states of solute and solvent table.
Answer:
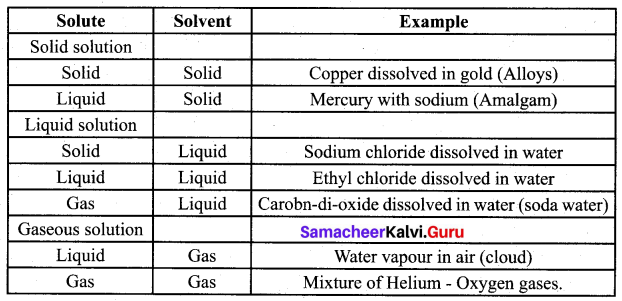
(b) State Henry’s law?
Answer:
Henry’s law states that, the solubility of a gas in a liquid, is directly proportional to the pressure of the gas over the solution at a definite temperature.
![]()
Question 28.
(a) Give an example for conditioned reflexes.
Answer:
Playing harmonium by striking a particular key, on seeing a music note is an example of conditioned reflexes which required conscious training effort.
(b) How many cranial nerves and spinal nerves are present in man?
Answer:
12 pairs of cranial nerves and 31 pairs of spinal nerves are present in man.
Question 29.
(a) What are Allosomes?
Answer:
Allosomes are chromosomes which are responsible for determining the sex of an individual. There are two types of sex chromosomes. X and Y chromosomes. Human male have one X chromosome and one Y chromosomes and the human female have two X chromosomes.
(b) What causes the opening and closing of guard cells of stomata during transpiration?
Answer:
The opening and closing of stomata is due to the change in turgidity of the guard cells. When the water enters into the guard cells, they become turgid and the stomata open. When guard cells lose water, it becomes flaccid and the stomata closes.
![]()
Question 30.
(a) Draw and label the structure of oxysomes
Answer:
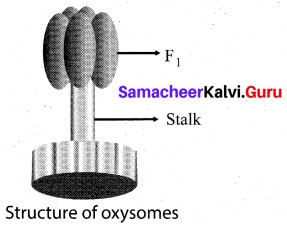
(b) What is respiratory quotient?
Answer:
The ratio of volume of carbon dioxide liberated and the volume of oxygen consumed, during respiration is called Respiratory Quotient (R.Q)

Question 31.
(a) Why is a dietary restriction recommended for an obese individual?
Answer:
Obesity is a positive risk factor in development of hypertension, diabetes, gall bladder disease, coronary heart disease and arthritis. Hence dietary restriction is recommended for an obese individual.
(b) Give the name of wheat variety having higher dietary fibre and protein.
Answer:
Triticale (6n) is a hybrid of wheat and rye. It has higher dietary fibre and protein.
Question 32.
(a) State Newton’s third law of motion and explain with examples:
Answer:
Law: ‘For every action, there is an equal and opposite reaction.
They always act on two different bodies’.
FB = – FA
Examples:
(i) When birds fly:
Push the air downwards with their wings – Action
Air push the bird upwards – Reaction
(ii) When firing bullet:
Gun recoils backward and the bullet is moving forward – Action
Gun equals this forward action by moving backward – Reaction.
(b) How will you identify saturated and unsaturated compounds?
Answer:
- Take the given sample solution in a test tube.
- Add a few drops of bromine water
- If the given compound is unsaturated, it will decolourise bromine water.
- If the given compound is saturated it will not decolourise bromine water.
Part – IV
(1) Answer all the questions. [3 × 7 = 21]
(2) Each question carries seven marks.
(3) Draw diagram wherever necessary.
Question 33.
(a) (i) State Rayleigh’s law of scattering.
Answer:
Rayleigh’s scattering law states that “The amount of scattering of light is inversely proportional to the fourth power of its wavelength”.
Amount of scattering, S ∝ \(\frac{1}{\lambda^{4}}\)
(ii) Explain the construction and working of a ‘compound microscope’.
Answer:
Construction
A compound microscope consists of two convex lenses. The lens with the shorter focal length is placed near the object, and is called as ‘objective lens’ or ‘objective piece’. The lens with larger focal length and larger aperture placed near the observer’s eye is called as ‘eye lens’ or ‘eye piece’. Both the lenses are fixed in a narrow tube with adjustable provision.
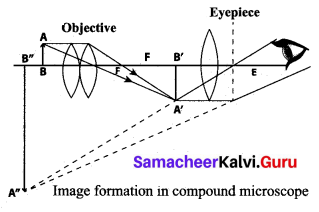
Working:
(i) The object (AB) is placed at a distance slightly greater than the focal length of objective lens (u >fo).
(ii) A real, inverted and magnified image (A’B’) is formed at the other side of the objective lens.
(iii) This image behaves as the object for the eye lens. The position of the eye lens is adjusted in such a way, that the image (A’B’) falls within the principal focus of the eye piece.
(iv) This eye piece forms a virtual, enlarged and erect image (A” B”) on the same side of the object.
(v) Compound microscope has 50 to 200 times more magnification power than simple microscope.
[OR]
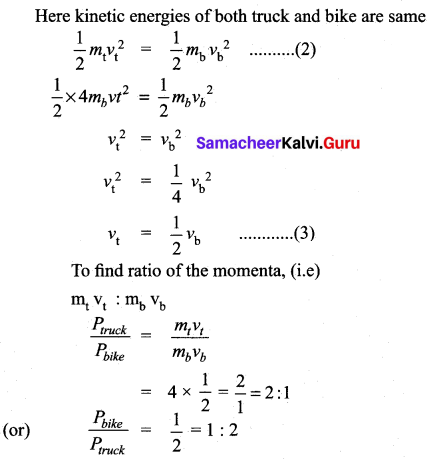
(b) (i) A heavy truck and bike are moving with the same kinetic energy. If the mass of the truck is four times that of the bike, then calculate the ratio of their momenta. (Ratio of momenta = 1:2)
Answer:
Given: Let mb , mt are the masses of truck and bike.
mt = 4mb ………(1)
(ii) Air temperature in the Rajasthan desert can reach 46°C. What is the velocity of sound in air at that temperature? (v0 = 331 ms-1)
Answer:
VT = (V0 + 0.16 T)
T = 46°C, v0 =331 ms-1
VT = 331 +(0.61 × 46)
= 331 +28.06
VT = 359.06 ms-1
![]()
Question 34.
(a) (i) A solution is prepared by dissolving 15 g of NaCl in 85 g of water. Calculate the mass percentage of solute.
Answer:
Mass of solute = 15 g
Mass of solvent = 85 g
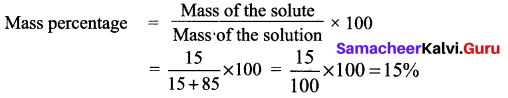
(ii) Calculate the pH of 0.001 M NaOH.
Answer:
[OH–] = Normality = Molarity × acidity
= 0.001 × 1 = 1 × 10-3
pOH = -log10 [OH–]
= -log10 1× [10-3]
= -log 1– log10 10-3
= 0 – (-3 log10 10)
pOH = 3
pH + pOH = 14 .
pH = 14 – pOH= 14 – 3
pH = 11
(iii) Calculate the mass of 0.01 mole of H2O.
Answer:
n = 0.01 mole; μ = 18 g mol-1; W = ?
W = n × M
= 0.01 × 18 = 0.18 g
[OR]
(b) (i) In what way hygroscopic substances differ from deliquescent substances.
Answer:
Difference between hygroscopic and deliquescent substances is in the extent to which each material can absorb moisture. This is because both of these terms are very much related to each other and they refer to the property of observing and the retention of moisture from the air. However, they differ in the extent of absorption of moisture where hygroscopic materials absorb moisture but not the extent the original substance dissolves in it, which is the casewith deliquescence. Therefore deliquescence can be regarded as an extreme condition of hygroscopic activity.
| Hygroscopic substances | Deliquescence substance |
| 1. When exposed to the atmosphere at ordinary temperature, they absorb moisture and do not dissolve. | 1. When exposed to the atmospheric air at ordinary temperature, they absorb moisture and dissolve. |
| 2. Hygroscopic substances do not change its physical state on exposure to air. | 2. Deliquescent substances change its physical state on exposure to air. |
| 3. Hygroscopic substances may be amorphous solids or liquids. | 3. Deliquescent substances are crystalline solids. |
(ii) Define solubility.
Answer:
It is defined as the number of grams of a solute that can be dissolved in 100 g of a solvent to form its saturated solution at a given temperature and pressure.
![]()
Question 35.
(a) (i) Which hormone is known as stress hormone in plants ? Why?
Answer:
Abscisic acid (ABA) is a growth, inhibitor which regulates abscission and dormancy. It increases tolerance of plants to various kinds of stress. So, it is also called as stress hormone. It is found in the chloroplast of plants.
(ii) How does binary fission differ from multiple fission?
Answer:
| Binary fission | Multiple fission |
| 1. Binary fission is the splitting of a cell into two daughter cell | 1. Multiple fission is splitting of a parent cell into two or more daughter cells. |
| 2. It takes place in favourable conditions. Eg. Amoeba |
2. It takes place in unfavourable conditions. Eg. Plasmodium |
(iii) What are the sources of solid wastes? How are solid wastes managed?
Answer:
Solid wastes mainly include municipal wastes, hospital wastes, industrial wastes and e-wastes, etc.
Methods of solid wastes disposal:
(1) Segregation: It is the separation of different type of waste materials like biodegradable and non-biodegradable wastes.
(2) Sanitary landfill: Solid wastes are dumped into low lying areas. The layers are compacted by trucks to allow settlement. The waste materials get stabilised in about 2-12 months. The organic matter undergoes decomposition.
(3) Incineration: It is the burning of non-biodegradable solid wastes (medical wastes) in properly constructed furnace at high temperature.
(4) Composting: Biodegradable matter of solid wastes is digested by microbial action or earthworms and converted into humus.
[OR]
(b) (i) Explain the male reproductive system of rabbit?
Answer:
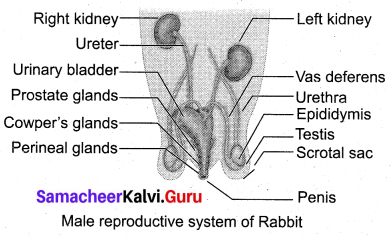
The male reproductive system of rabbit consists of a pair of testes which are ovoid in shape. Testes are enclosed by scrotal sacs in the abdominal cavity. Each testis consists of numerous fine tubules called seminiferous tubules.
This network of tubules lead into Cowper s glands a coiled tubule called epididymis, Perineal glands which lead into the sperm duct called vas deferens.
The vas deferens join in the urethra just below the urinary bladder. The urethra runs backward and passes into the penis. There are three accessory glands namely prostate gland, cowper’s gland and perineal gland. Their secretions are involved in reproduction.
(ii) What are vestigeal organs?
Answer:
The degenerated and non functional organs of animals are called Vestigeal organs. Eg. Vermiform appendix, nictitating membrane, caudal vertebra, coccyx.