Students can Download Samacheer Kalvi 10th Science Model Question Paper 5 English Medium Pdf, Samacheer Kalvi 10th Science Model Question Papers helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamil Nadu Samacheer Kalvi 10th Science Model Question Paper 5 English Medium
General Instructions:
- The question paper comprises of four parts
- You are to attempt all the questions in each part. An internal choice of questions is provided wherever applicable.
- All questions of Part I, II, III and IV are to be attempted separately.
- Question numbers 1 to 12 in Part I are Multiple Choice Questions of one mark each.
These are to be answered by writing the correct answer along with the corresponding option code. - Question numbers 13 to 22 in Part II are of two marks each. Any one question should be answered compulsorily.
- Question numbers 23 to 32 in Part III are of four marks each. Any one question should be answered compulsorily.
- Question numbers 33 to 35 in Part IV are of seven marks each. Draw diagrams wherever necessary.
Time: 3 Hours
Maximum Marks: 75
Part – I
(i) Answer all the questions. [12 × 1 = 12]
(ii) Choose the most suitable answer and write the code with the corresponding answer.
Question 1.
To project the rockets which of the following principle(s) is /(are) required?
(a) Newton’s third law of motion
(b) Newton’s law of gravitation
(c) Law of conservation of linear momentum
(d) Both (a) and (c)
Answer:
(d) Both (a) and (c)
Question 2.
Kilowatt hour is the unit of _______.
(a) resistivity
(b) conductivity
(c) electrical energy
(d) electrical power
Answer:
(c) electrical energy
Question 3.
_______ element emits its radiation spontaneously.
(a) Ni
(b) Pb
(c)Pt
(d) u
Answer:
(d) u
![]()
Question 4.
Which of the following is a triatomic molecule?
(a) Glucose
(b) Helium
(c) Carbon-di-oxide
(d) Hydrogen
Answer:
(c) Carbon-di-oxide
Question 5.
________ is an important metal to form amalgam.
(a) Ag
(b) Hg
(c) Mg
(d) Al
Answer:
(b) Hg
Question 6.
The component present in lesser amount, in a solution is called _______.
(a) solute
(b) solvent
(c) solution
(d) colloid
Answer:
(a) solute
Question 7.
A patient with blood group ‘O’ was injured in an accident and has blood loss. Which blood group the doctor should effectively use _______ for transfusion.
(a) O group
(b) AB group
(c) A or B group
(d) All blood group
Answer:
(a) O group
![]()
Question 8.
Excessive consumption of alcohol leads to _______.
(a) Loss of memory
(b) Cirrhosis of liver
(c) State of hallucination
(d) Suppression of brain function
Answer:
(b) Cirrhosis of liver
Question 9.
_______ is formed during anaerobic respiration.
(a) Carbohydrate
(b) Ethyl alcohol
(c) Acetyl CoA
(d) Pyruvate
Answer:
(b) Ethyl alcohol
Question 10.
Casparian strips are present in the _____ of the root.
(a) Cortex
(b) Pith
(c) Pericycle
(d) Endodermis
Answer:
(d) Endodermis
Question 11.
The soft finely stratified sedimentary rock refer to _______.
(a) Shale
(b) Petroleum
(c) Methane
(d) Coal
Answer:
(a) Shale
![]()
Question 12.
All files are stored in the _______.
(a) Folder
(b) Box
(c) Paint
(d) Scanner
Answer:
(a) Folder
Part – II
Answer any seven questions. (Q.No: 22 is compulsory) [7 × 2 = 14]
Question 13.
State Newton’s second law.
Answer:
“The force acting on a body is directly proportional to the rate of change of linear momentum of the body and the change in momentum takes place in the direction of the force”.
Question 14.
What are the caused of “Myopia”?
Answer:
Myopia, also known as short sightedness, occurs due to the lengthening of eye ball. With this defect, nearby objects can be seen clearly but distance objects cannot be seen clearly.
![]()
Question 15.
What is the minimum distance needed for an echo?
Answer:
- The minimum distance required to hear an echo is 1/20th part of the magnitude of the velocity of sound in air.
- If you consider the velocity of sound as 344 ms-1, the minimum distance required to hear an echo is 17.2 m.
Question 16.
Why does the reaction rate of a reaction increase on raising the temperature?
Answer:
On increasing temperature heat is supplied to the reactant. This energy breaks more bonds and thus speed up the chemical reaction. Foods kept at room temperature spoils faster than that kept in the refrigerator.
Question 17.
Differentiate soaps and detergents.
Answer:
| Soaps | Detergents |
| 1. It is a sodium salt of long chain fatty acids. | 1. It is a sodium salt of sulphonic acids. |
| 2. Its effectiveness is reduced when used in hard water. | 2. It is effective even in hard water. |
| 3. Soaps are biodegradable. | 3. Most of the detergents are non-biodegradable. |
Question 18.
Write the dental formula of rabbit.
Answer:
Dental formula is (I \(\frac{2}{1}\), C\(\frac{0}{0}\), PM \(\frac{2}{1}\), M \(\frac{2}{1}\)) in Rabbit, which is written as \(\frac{2033}{1023}\)
I – Incisors, C – Canine, PM – Premolar and M-molar.
![]()
Question 19.
What is bolting? How can it be induced artificially?
Answer:
Sudden shoot elongation followed by flowering is known as bolting. Artifically bolting can be induced on rosette plants by the treatment of Gibberellins.
Question 20.
Identify the parts A,B,C & D?
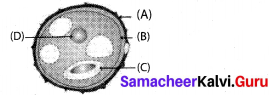
Answer:
A – Exine
B – Intine
C – Generative cell
D – Vegetative nucleus
Question 21.
How can you determine the age of the fossils?
Answer:
The age of fossils is determined by radioactive elements present in it. They may be carbon, uranium, lead or potassium. By radio active carbon (C14) dating method, carbon consumption of animals and plants stops after death and since then, only the decaying process of C14 occurs continuously. The time passed since death of a plant or animal can be calculated by measuring the amount of C14 present in their body.
Question 22.
A charge of 10 coulomb flows through a bulb in 5 second. What is the current through the bulb?
Answer:
Given : Q = 10 C
t = 5 s
I = \(\frac{Q}{t}=\frac{10}{5}\)
I = 2 A
Part – III
Answer any seven questions (Q.No: 32 is compulsory) [7 × 4 = 28]
Question 23.
State and prove the law of conservation of linear momentum.
Answer:
(i) There is no change in the linear momentum of a system of bodies as long as no net external force acts on them.
(ii) Let us prove the law of conservation of linear momentum with the following illustration:
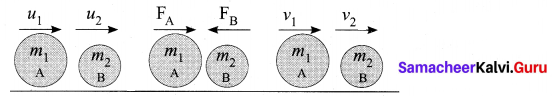
(iii) Let two bodies A and B having masses m1 and m2 move with initial velocity u1 and u2 in a straight line.
(iv) Let the velocity of the first body be higher than that of the second body. i.e., u1 > u2.
(v) During an interval of time t second, they tend to have a collision. After the impact, both of them move along the same straight line with a velocity v1 and v2 respectively.
Force on body B due to A, FB = \(\frac{m_{2}\left[v_{2}-u_{2}\right]}{t}\)
Force on body A due to B, FA = \(\frac{m_{1}\left[v_{1}-u_{1}\right]}{t}\)
By Newton’s III law of motion,
Action force = Reaction force
FA = -FB
\(\frac{m_{1}\left[v_{1}-u_{1}\right]}{t}=-\frac{m_{2}\left[v_{2}-u_{2}\right]}{t}\)
m1v1 – m1u1 = -m2v2 + m2u2
m1v1 + m2v2 = m1u1 + m2u2
The above equation confirms in the absence of an external force, the algebraic sum of the momentum after collision is numerically equal to the algebraic sum of the momentum before collision. Hence file law of conservation linear momentum is proved.
![]()
Question 24.
(i) Draw a ray diagram to show the image formed by a convex lens when the object is placed between F and 2F.
Answer:
Ray diagram for object placed between F and 2F
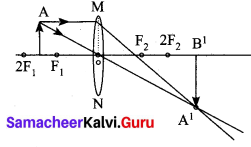
(ii) Define one calorie.
Answer:
One calorie is defined as the amount of heat energy required to rise the temperature of 1 gram of water through 1°C.
Question 25.
(i) How many electrons are passing per second in a circuit in which there is a current of 5A?
Answer:
Current I = 5 A
time (t) = 1 second
Charge of electron e = 1.6 × 10-19 C
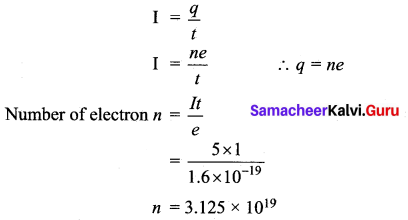
(ii) Mention two cases in which there is no Doppler effect in sound?
Answer:
- When source (S) and listener (L) both are at rest.
- When S and L move in such a way that distance between them remains constant.
- When source S and L are moving in mutually perpendicular directions.
- If the source is situated at the center of the circle along which the listener is moving.
Question 26.
Derive the relationship between Relative molecular mass and Vapour density.
Answer:
(а) The Relative Molecular Mass of a gas or vapour is the ratio between the mass of one molecule of the gas or vapour to mass of one atom of Hydrogen.
(b) Vapour density is the ratio of the mass of a certain volume of a gas or vapour, to the mass of an equal volume of hydrogen, measured under the same conditions of temperature and pressure.
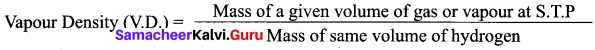
(c) According to Avogadro’s law, equal volumes of all gases contain equal number of molecules.
Thus, let the number of molecules in one volume = n, then
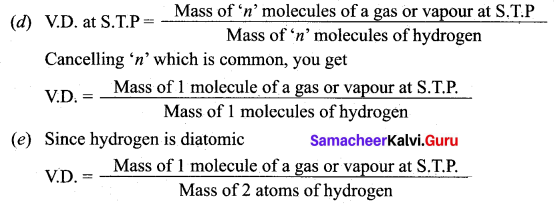
(f) By comparing the definition of relative molecular mass Mid vapour density we can write as follows.

(g) By substituting the relative molecular mass value in vapour density definition, we get Vapour density (V.D.) = Relative molecular mass / 2
2 × vapour density = Relative molecular mass of a gas
![]()
Question 27.
How is metal corrosion prevented?
Answer:
Methods of preventing corrosion:
1. Alloying: The metals can be alloyed to prevent the process of corrosion.
E.g: Stainless Steel
2. Surface Coating: It involves application of a protective coating over the metal. It is of the
following types:
(a) Galvanization: It is the process of coating zinc on iron sheets by using electric current.
(b) Electroplating: It is a method of coating one metal over another metal by passing electric current.
(c) Anodizing: It is an electrochemical process that converts the metal surface into a decorative, durable and corrosion resistant. Aluminium is widely used for anodizing process.
(d) Cathodic Protection: It is the method of controlling corrosion of a metal surface protected is coated with the metal which is easily corrodible. The easily corrodible metal is called Sacrificial metal to act as anode ensuring cathodic protection.
Question 28.
With a neat labelled diagram explain the structure of a neuron.
Answer:
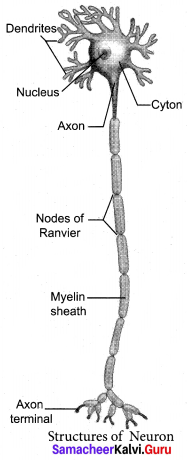
A typical neuron consists of 3 parts: Cyton, Dendrites and Axon.
(1) Cyton:
- It has a central nucleus with the abundant cytoplasm called neuroplasm.
- The cytoplasm has large granular body called Nissel’s granules
- Other cell organelles like mitochondria, ribosomes, lysosomes, and endoplasmic reticulum are present.
- Several neurofibrils in the cytoplasm help in transmission of nerve impulses to and form the cell body.
(2) Dendrites:
- These are the numerous branched cytoplasmic processes, that project from the surface of the cell body.
- They conduct nerve impulses, towards the cyton.
- The branched projections increase the surface area for receiving the signals from other nerve cells.
(3) Axon:
- It is a single, elongated, slender projection.
- The end of axon terminates as fine branches, which terminate into knob like swellings called synaptic knob.
- The plasma membrane of axon is called axolemma,
- The cytoplasm of axon is called axoplasm.
- Axon carries impulses away from the cyton.
- The protective sheath around the axon is myelin sheath
- The myelin sheath breaks at intervals by depression called Nodes of Ranvier. It acts as insulator and ensures rapid transmission of nerve impulses.
- The junction between two neuron is called synapsis.
Question 29.
(i) Read the following content and answer the questions below:
Pure-bred tall pea plants are first crossed with pure-bred dwarf pea plants. The pea plants obtained in F1 generation are then cross-bred to produce F2 generation of pea plants.
(a) What do the plants of F1 generation look like?
(b) What is the ratio of tall plants to dwarf plants in F2 generation?
(c) Which type of plants were missing in F1 generation but reappeared in F2 generation?
Answer:
(a) In F1 generation, all plants are tall (Tt)
(b) In F2 generation the ratio of tall plants to dwarf plant is 3:1. i.e., 3 tall and 1 dwarf
Three types of plants were obtained
Tall homozygous : TT – 1
Tall heterozygous : Tt – 2
Dwarf homozygous : tt -1
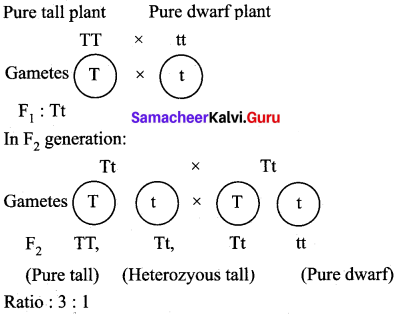
(c) Dwarf plants were missing on F1 but reappeared in F2 generation.
(ii) Why is the sino-atrial node Called the pacemaker of heart?
Answer:
Sino-atrial node acts as the ‘pacemaker’ of the heart because it is capable of initiating impulse which can stimulate the heart muscles to contract.
![]()
Question 30.
Differentiate the following:
(a) Monocot root and Dicot root
(b) Aerobic and Anaerobic respiration (a) Monocot root and Dicot root
Answer:
| Monocot Root | Dicot Root |
| 1. Number of Xylem Polyarch | 1. Tetrach |
| 2. Cambium is absent. | 2. Cambium is present during secondary growth |
| 3. Secondary growth absent. | 3. Secondary growth present. |
| 4. Pith present | 4. Pith absent |
(b) Aerobic and Anaerobic respiration
Answer:
| Aerobic respiration | Anaerobic respiration |
| 1. Occur in the presence of oxygen. | 1. Occurs in the absense of oxygen |
| 2. Carbon dioxide, water and ATP are produced. | 2. Lactic acid, Ethanol and ATP are produced. |
| 3. It occurs in most plant and animals | 3. It occurs in plants and bacteria |
Question 31.
(i) What precautions can be taken for preventing heart disease?
(ii) Name two maize hybrids rich in amino acid lysine.
Answer:
(i) Prevention for preventing heart disease
- Reduction in the intake of calories, low saturated fat, cholesterol rich food, low carbohydrates and common salt.
- Increase in the intake of fibre diet, fruits and vegetables, protein, minerals and vitamins.
- Regular exercise, walking and yoga are essential for body weight maintenance.
- Avoid alcohol consumption, Psychotrophic drugs and smoking.
(ii) Protina, shakt and Rathna are lysine rich maize hybrids, which are developed in India.
Question 32.
(i) Write the features of nuclear fission and nuclear fusion.
Answer:
Nuclear Fission
- The process of breaking up of a heavy nucleus into two smaller nuclei is called ‘nuclear fission’.
- Can be performed at room temperature.
- Alpha, beta and gamma radiations are emitted.
- Fission leads to emission of gamma radiation. This triggers the mutation in the human gene and causes genetic transform diseases.
Nuclear Fusion:
- Nuclear fusion is the combination of two lighter nuclei to form a heavier nucleus.
- Extremely high temperature and pressure is needed.
- Alpha rays, positrons, and neutrinos are emitted.
- Only light and heat energy is emitted.
(ii) Calculate the pH of 0.01 M HNO3?
Answer:
pH = -log[H+]
= -log10 -2
= (-2) (- log1010)
= 2 × 1.00
pH = 2
Part – IV
(1) Answer all the questions. [3 × 7 = 21]
(2) Each question carries seven marks.
(3) Draw diagram wherever necessary.
Question 33.
(i) State Boyle’s law.
Answer:
When the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
P ∝ 1/V
(ii) Explain the experiment of measuring the real and apparent expansion of a liquid with a neat diagram.
Answer:
(1) To start with, the liquid whose real and apparent expansion is to be determined is poured in a container up to a level. Mark this level as L1.
(2) Now, heat the container and the liquid using a burner as shown in the figure Initially, the container receives the thermal energy and it expands.
(3) As a result, the volume of the liquid appears to have reduced. Mark this reduced level of liquid as L2.
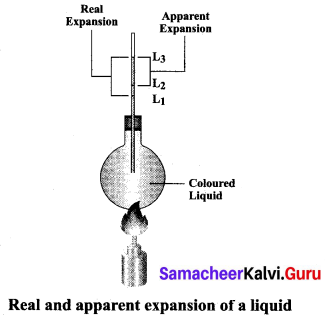
On further heating, the thermal energy supplied to the liquid through the container results in the expansion of the liquid. Hence, the level of liquid rises to L3. Now, the difference between the levels L1 and L3 is called as apparent expansion, and the difference between the levels L2 and L3 is called real expansion. The real expansion is always more than that of apparent expansion.
Real expansion = L3 – L2
Apparent expansion = L3 – L1
[OR]
(b) (i) A source of sound is moving with a velocity of 50 ms-1 towards a stationary listener. The listener measures the frequency of the source as 1000 Hz. what will be the apparent frequency of the source when it is moving away from the listener after crossing him? (velocity of sound in the medium is 330 ms-1)
Answer:
When the source is moving towards the stationary listener, the expression for apparent frequency is
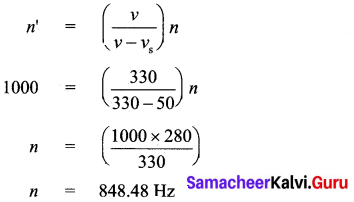
The actual frequency of the sound is 848.48 Hz. When the source is moving away from the stationary listener, the expression for apparent frequency is
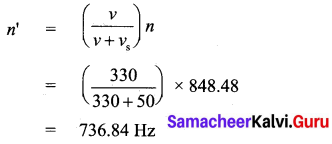
(ii) Calculate the velocity of a moving body of mass 5 kg whose linear momentum is 2 kg ms-1.
Answer:
Linear momentum = mass × velocity
Velocity = Linear momentum / mass
v = 2.5 / 5 = 0.5 ms-1
![]()
Question 34.
(a) (i) Calculate the mass of 1.51 x 10-23 molecule of H2O.
Answer:
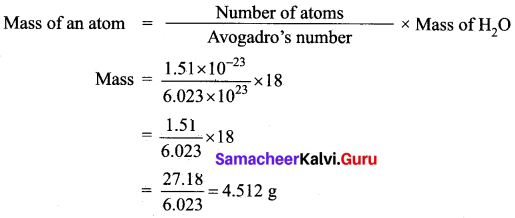
(ii) Calculate the moles of 46 g sodium.
Answer:
46 g of sodium
Atomic mass of sodium = 23
Mass of sodium = 46
No. of moles = \(\frac{\text { mass }}{\text { atomic mass }}=\frac{46}{23}\)
= 2 mole of sodium
(iii) Calculate the number of molecules present in the 36 g water.
Answer:
H2O → = (1 × 2) + (1 × 16)
= 2 + 16
H2O = 18 g

= 12.046 × 1023 atoms
36 g water contains → 12.046 x 1023 atoms
[OR]
(b) (i) Write notes on various factors affecting solubility.
Answer:
Factors affecting solubility: There are three main factors which govern die solubility of solute. They are:
- Nature of the solute arid solvent
- Temperature
- Pressure
(1) Nature of the solute and solvent: The nature of the solute and solvent plays an important role in solubility. Although water dissolves an enormous variety of substances, both ionic and covalent, it does not dissolve everything.
The phrase that scientists often use when predicting solubility is “like dissolves like.” This expression means that dissolving occurs when similarities exist between the solvent and the solute. For example: Common salt is a polar compound and dissolves readily in polar solvent like water.
Non-polar compounds are soluble in non-polar solvents. For example, Fat dissolved in ether. But non-polar compounds, do not dissolve in polar solvents; polar compounds do not dissolve in non-polar solvents.
(2) Effect of Temperature:
Solubility of Solids in Liquid: Generally, solubility of a solid solute in a liquid solvent increases with increase in temperature. For example, a greater amount of sugar will dissolve in warm water than in cold water.
In endothermic process, solubility increases with increase in temperature.
In exothermic process, solubility decreases with increase in temperature.
Solubility of Gases in liquid : Solubility of gases in liquid decrease with increase in temperature. Generally, water contains dissolved oxygen. When water is boiled, the solubility of oxygen in water decreases, so oxygen escapes in the form of bubbles.
Aquatic animals live more in cold regions because, more amount of dissolved oxygen is present in the water of cold regions. This shows that the solubility of oxygen in water is more at low temperatures.
(3) Effect of Pressure: Effect of pressure is observed only in the case of solubility of a gas in a liquid. When the pressure is increased, the solubility of a gas in liquid increases.
The common examples for solubility of gases in liquids are carbonated beverages, i.e. soft drinks, household cleaners containing aqueous solution of ammonia, formalinaqueous solution of fonnaldehyde, etc.
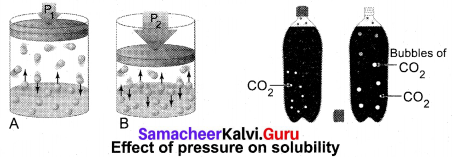
![]()
Question 35.
(a) (i) Why are thyroid hormones referred as personality hormone?
(ii) Define triple fusion?
(iii) Enumerate the importance of forests?
Answer:
(i) Thyroid hormones is essential for normal physical, mental and personality development. Hence it is called as personality honnone.
(ii) The fusion involving two polar nucleus and a sperm nucleus, that occurs in double fertilization in a seed plant and results in the formation of endosperm, is called the triple fusion.
(iii) Importance of forests.
- Forests are the important component of our environment and are dominated by microorganisms, flowering plants, shrubs, climbers and dense trees.
- Forests provide a vast habitat for wild animals.
- Forests help for the economic development of our country.
- Forests are the source for a wide range of renewable natural resource.
- Forests provide wood, food, fodder, fibre and medicine.
- They act as carbon sink, regulate climatic conditions, increase rainfall and reduce global warming.
- Forests protect wild life and also acts as catchment for water conservation.
- They prevent natural hazards like floods and landslides
- They play a vital role in maintaining the ecological balance.
[OR]
(b) (i) List out any three parasitic adaptations in leech.
(ii) Natural selection is a driving force for evolution-How?
Answer:
(i) Parasitic adaptation of leech:
- Anterior and Posterior ends of the body are provided with suckers by which the animal attaches itself to the body of the host.
- The three Jaws, inside the mouth, causes a painless Y – shaped wound in the skin of the host.
- The salivary glands produce Hirudin, which does not allow the blood to coagulate. So, the continuous supply of blood is maintained.
(ii) Darwin published his observations under the name “origin of species”. It elaborates on the theory of natural selection for evolutionary transformation. The principles of Darwinism tells that natural selection is a driving force for evolution.
1. Overproduction: Living beings have the ability to reproduce and have the capacity to multiply in a geometrical manner.
2. Struggle for existence: Due to overproduction a geometric ratio of increase in population occurs. The space to live and food available for the organisms remain the same. This creates a competition among the organisms, for food and space, leading to struggle.
- The competition may be among the individuals of the same species (Intraspecific struggle).
- Competition between the organisms of different species living together (Interspecific struggle).
- Natural conditions like extreme heat or cold drought and floods can affect the existence of organisms (Environmental struggle).
3. Variations:
Small variations are important for evolution. According to Darwin, favourable variations are useful to the organisms and unfavourable variations are harmful or useless to the organisms.
4. Survival of the fittest or Natural selection:
During the struggle for existence, the organisms which can overcome the challenging situation,survive and adapt to the surrounding environment. Organisms, which are unable to face the challenges, are unfit to survive and disappear. The process of selection of organisms with favourable variation is called as Natural selection.
5. Origin of species:
According to Darwin, new species originates by the gradual accumulation of favourable variations for a number of generations.