The Samacheer Kalvi Class 11th Chemistry Solutions Chapter 15 Environmental Chemistry Questions and Answers prevailing here are designed by the academic subject expertise in accordance with the state board prescribed syllabus and books. Aspirants who require help in preparing the Tamilnadu State Board Class 11th Chemistry Solutions Chapter 15 Environmental Chemistry exercise questions can rely on the Samacheer Kalvi 11th Chemistry Solutions pdf for Chapter 15 Environmental Chemistry given in this article.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 15 Environmental Chemistry
Students who are excited for Tamilnadu State Board for Class 11th Chemistry Solutions Chapter 15 Environmental Chemistry can find detailed and step wise solutions for all questions of Chapter 15 Environmental Chemistry from here. Simply tap on the links available over here for which you have to view/download the Samacheer Kalvi Class 11th Solutions for Chemistry Solutions chapter 15 Environmental Chemistry Questions and Answers. All these solutions are free to download and easy to access online or offline so that you can prepare well for the exams at any time.
Samacheer Kalvi 11th Chemistry Environmental Chemistry Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Environmental Chemistry Multiple Choice Questions
Question 1.
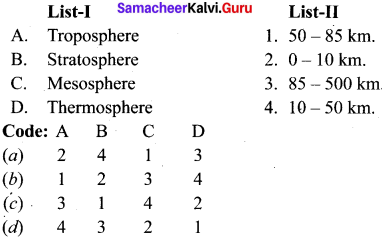
Th gaseous envelope around the earth is known as atmosphere. The region lying between an altitude of 11-50 km is –
(a) Troposphere
(b) Mesophere
(c) Thermosphere
(d) Stratosphere
Answer:
(d) Stratosphere
Question 2.
Which of the following is natural and human disturbance in ecology?
(a) Forest fire
(b) Floods
(c) Acid rain
(d) Greenhouse effect
Answer:
(a) Forest fire
Question 3.
Bhopal Gas Tragedy is a case of –
(a) thermal pollution
(b) air pollution
(c) nuclear pollution
(d) land pollution
Answer:
(b) air pollution
Question 4.
Haemoglobin of the blood forms carboxyhaemoglobin with –
(a) Carbon dioxide
(b) Carbon tetrachioride
(c) Carbon monoxide
(d) Carbonic acid
Answer:
(c) Carbon monoxide
Question 5.
Which sequence for greenhouse gases is based on GWP?
(a) CFC > N2O > CO2 > CH4
(b) CFC > CO2 > N2O > CH4
(c) CFC > N2O > CH4 > CO2
(d) CFC > CH4 > N2O > CO2
Answer:
(c) CFC > N2O > CH4 > CO2
Question 6.
Photo chemical smog formed in congested metropolitan cities mainly consists of –
(a) Ozone, SO2 and hydrocarbons
(b) Ozone, PAN and NO2
(c) PAN, smoke and SO2
(d) Hydrocarbons, SO2 and CO2
Answer:
(b) Ozone, PAN and NO2
Question 7.
The pH of normal rain water is –
(a) 6.5
(b) 7.5
(c) 5.6
(d) 4.6
Answer:
(c) 5.6
Question 8.
Ozone depletion will cause –
(a) forest fires
(b) eutrophication
(c) bio magnification
(d) global warming
Answer:
(d) global warming
Question 9.
Identify the wrong statement in the following.
(a) The clean water would have a BOD value of more than 5 ppm
(b) Greenhouse effect is also called as Global warming
(c) Minute solid particles in air is known as particulate pollutants
(d) Biosphere is the protective blanket of gases surrounding the earth
Answer:
(a) The clean water would have a BOD value of more than 5 ppm
Question 10.
Living in the atmosphere of CO is dangerous because it –
(a) Combines with O2 present inside to form CO2
(b) Reduces organic matter of tissues
(c) Combines with haemoglobin and makes it incapable to absorb oxygen
(d) Dries up the blood
Answer:
(c) Combines with haemoglobin and makes it incapable to absorb oxygen
Question 11.
Release of oxides of nitrogen and hydrocarbons into the atmosphere by motor vehicles is prevented by using –
(a) grit hamber
(b) scrubbers
(c) trickling filters
(d) catalytic convertors
Answer:
(c) trickling filters
Question 12.
Biochemical oxygen Demand value less than 5 ppm indicates a water sample to be
(a) highly polluted
(b) poor in dissolved oxygen
(c) rich in dissolved oxygen
(d) low COD
Answer:
(c) rich in dissolved oxygen
Question 13.
Match the list I and list II and select the correct answer using the code given below in the list:
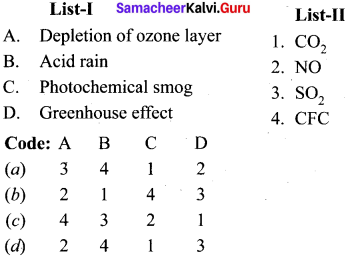
Answer:
![]()
Question 14.
Match the list I and list II and select the correct answer using the code given below in the list
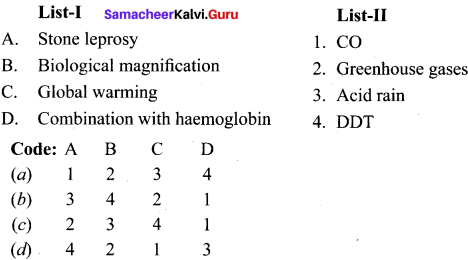
Answer:
![]()
Question 15.
Assertion (A) : if BOD level of water in a reservoir is more than 5 ppm it is highly polluted.
Reason(R) : High biological oxygen demand means high activity of bacteria in water
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Answer:
(d) (iv)
Question 16.
Assertion (A) : Excessive use of chlorinated pesticide causes soil and water pollution.
Reason (R) : Such pesticides arc non-biodegradable.
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Answer:
(a) (i)
Question 17.
Assertion (A) : Oxygen plays a key role in the troposphere.
Reason (R) : Troposphere is not responsible for all biological activities
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Answer:
(d) (iv)
Samacheer Kalvi 11th Chemistry Environmental Chemistry Short Answer Questions
Question 18.
Dissolved oxygen in water is responsible for aquatic life. What processes are responsible for the reduction in dissolved oxygen in water?
Answer:
The process which is responsible for the reduction of dissolved oxygen in water is excessive use of phosphatic and nitrate fertilizers, detergents, the discharge of human sewage and organic waste from food, paper, and pulp industries. The microorganisms which oxidize organic matter also used oxygen dissolved in H2O.
Moreover, during the night, photosynthesis stops but the aquatic plants continue to respire, resulting in a reduction of dissolved oxygen.
Question 19.
What would happen, if the greenhouse gases were totally missing in the earth’s atmosphere?
Answer:
- The primary greenhouse gases in Earth’s atmosphere are water vapour, carbon dioxide, methane, nitrous oxide and ozone.
- Naturally occurring greenhouse gases allow solar radiations to reach the earth’s surface, while trapping radiations from the earth on its way back out to space.
- There would he no life on Earth without the warmth provided by this natural greenhouse gases.
- In the absence of greenhouse gases. the average temperature of the earth will decrease drastically. As a result. life on Earth would be impossible.
Question 20.
Define smog.
Answer:
Smog is a combination of smoke and fog which forms droplets that remain suspended in the air. Smog is a chemical mixture of gases that forms a brownish-yellow haze over urban cities. Smog mainly consists of ground-level ozone, oxides of nitrogen, volatile organic compounds, SO2 acidic aerosols and gases, and particulate matter.
Question 21.
Which is considered to be earth’s protective umbrella? Why?
Answer:
- At high altitudes in the atmosphere consists of a layer of ozone (O2) which acts as an umbrella for harmful UV radiations. Ozone is considered to be earth’s protective umbrella.
- It protects us from harmful effects of UV-radiations of the sun such as skin cancer.
- Ozone layer prevent the UV radiations to reach the earth surface. So it acts as an umbrella for the Earth.
Question 22.
What are bio-degradable and non-biodegradable pollutants?
Answer:
- The pollutants which can be easily decomposed by the natural biological processes are called biodegradable pollutants. For example plant wastes, animal wastes.
- The pollutants which cannot be decomposed by the natural biological processes are called non-biodegradable pollutants. For example, metal wastes such as Hg and Pb, D.D.T. plastics, nuclear vastcs.
Question 23.
From where does ozone come in the photochemical smog?
Answer:
- Photochemical smog is formed by the combination of smoke, dust and fog with air pollutants in the presence of sunlight.
- Chemically it is oxidising in nature because of high concentration of oxidising agents such as NO2 and O2. So it is also called oxidising smog.
- Photochemical smog is formed by following reactions:
- N2 + O2 → 2NO
2NO + O2 → 2NO2
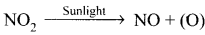
(O) + O2 → O3
O3 + NO → NO2 + O2
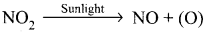
- NO and O3 arc strong oxidizing agents and they can react with unburnt hydrocarbons in polluted air to form formaldehyde, acrolcin and PAN.
Question 24.
A person was using water supplied by corporation. Due to shortage of water he started using underground water. He felt laxative effect. What could be the cause?
Answer:
A moderate concentration of sulphate ions in water are harmless but excessive concentration is greater than 500 ppm in water causes laxative effects. Hence under ground water may have consisted excess of suplhates.
Question 25.
What is green chemistry?
Answer:
- Green chemistry is a chemical philosophy encouraging the design of products and processes that reduces or eliminates the use and generation of hazardous substances.
- Efforts to control environmental pollution resulted in development of science for the synthesis of chemicals favorable to environment.
- Green chemistry means science of environmentally favorable chemical synthesis.
Question 26.
Explain how does greenhouse effect cause global warming.
Answer:
- The earth’s atmosphere allows most of the visible light from the sun to pass through and reach the earth’s surface. As earth’s surface is heated by sunlight, it radiates a part of this energy back towards the space as longer IR wavelengths.
- Some of the heat is trapped by CH2, CO2. CFCs and water vapour present in the atmosphere. They absorb IR radiations and block a large portion of earth’s emitted radiations.
- The radiations thus absorbed is partly remitted to the earth’s surface. Therefore the earth’s surface gets heated up by a phenomenon called greenhouse effect.
- Thus greenhouse effect is defined as the heating up of the earth surface due to trapping of infrared radiations reflected by earth’s surface by CO2 layer in the atmosphere. The heating up of the earth through the greenhouse effect is called global warming.
Question 27.
Mention the standards prescribed by BIS for quality of drinking water.
Answer:
Standard characteristics prescribed for deciding the quality of drinking water by BIS are as follows:
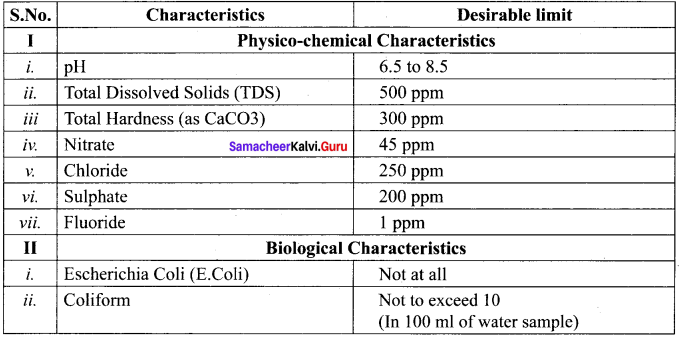
Question 28.
How does classical smog differ from photochemical smog?
Answer:
Classical smog was first observed in London in December 1952 and hence it is also known as Londo coal smoke and fog.
It occurs in cool atmospheric smog found in many large cities. The chemical composition is the mixture of SO2, SO3 and humidity. It generally occurs in the morning and becomes worse when the sun rises. This is mainly due to the induced oxidation of SO2 to SO3, which reacts with water yielding sulphuric acid aerosol.
Chemically it is reducing in nature because of the high concentration of SO2 and so it is also called reducing smog.
ii) Photochemical smog or Los Angel Smog:
Photo Chemical smog was first observed in Los Angels in 1950. It occurs in warm, dry, and sunny climates. This type of smog is formed by the combination of smoke, dust, and fog with air pollutants like oxides of nitrogen and hydrocarbons in the presence of sunlight.
It forms when the sun shines and becomes worse in the afternoon. Chemically it is oxidizing in nature because of the high concentration of oxidizing agents NO2 and O3, so it is also called oxidizing smog.
Question 29.
What are particulate pollutants? Explain any three.
Answer:
1. Particulate pollutants are small solid particles, and liquid droplets suspended in air.
Examples:
dust, pollen, smoke, soot and liquid aerosols.
2. Types of Particulates:
Particulates in the atmosphere may be of two types:
- viable particulate and
- non-viable particulate.
3.The viable particulates are small size living organisms such as bacteria, fhngi, moulds and algae which are dispersed in air.
4. The non-viable particulates are small solid particles and liquid droplets suspended in air. There are four types of non-viable particulates in the atmosphere. They are
(a) Smoke
(b) Dust
(c) Mist
(d) Fumes
5. Smoke:
Smoke particulate consists of solid particles formed by combustion of organic matter. For example, cigarette smoke, oil smoke, smokes from burning of fossil fuels, garbage and dry leaves.
6. Dust:
It is composed of fine solid particles produced during crushing and grinding of solid materials. For example, sand from sand blasting, saw dust from wood works and fly ash from power generating units.
7. Mist:
They are formed by particles of sprayed liquids and condensation of vapours in air. For example, sulphuric acid mist, herbicides and insecticides sprays can form mists.
8. Fumes:
They are obtained by condensation of vapours released during sublimation, distillation, boiling and calcination and by several other chemical reactions.
For example:
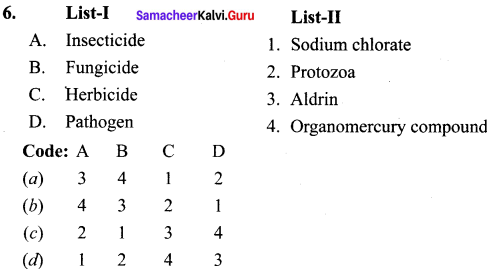
organic solvents, metals and metallic oxides.
Question 30.
Even though the use of pesticides increases the crop production, they adversely affect the living organisms. Explain the function and the adverse effects of the pesticides.
Answer:
Pesticides are chemicals that are used to kill or stop the growth of unwanted organisms. But these pesticides can affect the health of human beings.
These are further classified as
Insecticides:
Insecticides like DDT, BHC, aldrin etc. can stay in soil for long period of time and are absorbed by soil. They contaminate root crops like carrot, raddish, etc.
Fungicide:
Organo mercury compounds are used as most common fungicide. They dissociate in soil to produce mercury which is highly toxic.
Herbicides:
Herbicides are the chemical compounds used to control unwanted plants. They are otherwise known as weed killers. Example sodium chlorate (NaClO3) and sodium arsenite (Na3AsO3). Most of the herbicides are toxic to mammals.
Question 31.
Ethane bums completely in air to give CO2. while in a limited supply of air gives CO. The same gases are found in automobile exhaust. Both CO and CO2 are atmospheric pollutants
- What is the danger associated with these gases?
- How do the pollutants affect the human body?
Answer:
Danger associated with CO and CO2 & health hazards to human body
(a) Carbon monoxide binds with haemoglohin and form carboxyhaemoglobin which impairs normal oxygen transport by blood and hence the oxygen carrying capacity of blood is reduced. This oxygen deficiency results in headache, tension, dizziness, loss of consciousness, blurring of eyesight and cardiac arrest.
(b) Increase in CO2 level in the atmosphere is responsible for global warming. It causes headache and nausea.
Question 32.
On the basis of chemical reactions involved, explain how do CFC’s cause depletion of ozone layer in stratosphere?
Answer:
(i) The chioro-Iluoro derivatives of methane and ethane are named Freons (CFC’s). They slowly pass from troposphere to stratosphere. They stay for a very longer period of about 50-100 years. In the presence of UV radiations, CFC’s break up into chienne free radicals.
![]()
![]()
Cl• + O3 → { ClO }^{ \bullet } + O2
ClO• + O → { Cl }^{ \bullet } + O2
(iii) Chlorine radical is regenerated in the course of the reaction. Due to this continuous attack of Cl free radicals, thinning of ozone layer takes place which leads to the formation of ozone hole.
(iv) Li is estimated that for every reactive chlorine atom generated in the stratosphere 1,00,000 molecules of ozone are depleted.
Question 33.
How is acid rain formed? Explain its effect.
Answer:
Rainwater normally has a pH of 5.6 due to dissolution of atmospheric CO2 into it. Oxides, of sulphur and nitrogen in the atmosphere, may be absorbed by droplets of water that make up clouds and get chemically converted into sulphuric acid and nitric acid respectively as a results of pH of rainwater drops to the level 5.6 hence it is called acid rain.
Acid rain is a by-product of a variety of sulphur and nitrogen oxides in the atmosphere. Burning of fossil fuels (coal and oil) in power stations, furnaces and petrol, diesel in motor engines produce sulphur dioxide and nitrogen oxides. The main contributors to acid rain are SO2 and NO2. They are converted into sulphuric acid and nitric acid respectively by the reaction with oxygen and water.
2SO2 + O2 + 2H2O → 2H2SO4
4NO2 + O2 + 2H2O → 4HNO3
Question 34.
Differentiate the following:
- BOD and COD
- Viable and non-viable particulate pollutants
Answer:
Biochemical oxygen demand (BOD):
- The total amount of oxygen (in milligrams) consumed by microorganisms in decomposing the waste in one litre ut water at 20°C for a period of 5 days is called biochemical oxygen demand (BOD).
- its value is expressed in ppm.
- DOD is used as a measure of the degree of water pollution.
- BOD is only a measurement of consumed oxygen by microorganisms to decompose the organic matter.
Clean water would have BOD value less than 5 ppm.
Chemical ox gen demand (COD):
- Chemical oxygen demand is defined as the amount of oxygen required by the organic matter in a sample of water for its oxidation by a strong oxidising agent like K7Cr2O7 in acidic medium for a period of 2 hours.
- Its value is expressed in mg/litre.
- COD is a measure of amount of organic compounds in a water sample.
- COD refers to the requirement of dissolved oxygen for both the oxidation of organic and inorganic constituents.
- Clean water would have COD value greater than 250 mg/litre.
2. Viable and non-viable particulate pollutants
Viable pollutants:
- The viable particulates are small size living organisms such as bacteria, fungi. moulds, algae which are dispersed in air.
- They are all organic particulates.
- They contain living organisms.
- Eg. ftingi, bacteria, algae, moulds. Viable particles are the particles with at least one microorganism affecting the sterility of the product.
Non-viable pollutants:
- The non-viable particulates are small solid particles and liquid droplets suspended in air.
- They are all inorganic particulates.
- They contain non-living organisms.
- Eg. Smoke, dust, mist, fumes.
- Non-viable particles are the particles without microorganisms but act as transporting agent for viable particles.
Question 35.
Explain how oxygen deficiency is caused by carbon monoxide in our blood? Give its effect.
Answer:
Carbon monoxide is a poisonous gas produced as a result of incomplete combustion of coal are firewood. It is released into the air mainly by automobile exhaust. It binds with haemoglobin and forms carboxyhemoglobin which impairs normal oxygen transport by blood and hence the oxygen-carrying capacity of blood is reduced.
This oxygen deficiency results in headache, dizziness, tension, Loss of consciousness, blurring of eyesight, and cardiac arrest. Efforts to control environmental pollution have resulted in the development of science for the synthesis of chemicals favorable to the environment and it is called green chemistry.
Question 36.
What are the various methods you suggest lo protect our environment from pollution?
Answer:
- Waste management: Environmental pollution can be controlled by the proper disposal of wastes.
- Recycling: A large amount of disposed waste material can be reused by recycling the waste, thus it reduces the landfill and converts waste into useful forms.
- Substitution of less toxic solvents for highly toxic ones used in certain industrial processes.
- Use of fuels with lower sulphur content (e.g., washed coal)
- Growing more trees.
- Control measures in vehicle emissions are adequate.
- Efforts to control environmental pollution have resulted in the development of science for the synthesis of chemicals favourable to the environment and it is called green chemistry.
Samacheer Kalvi 11th Chemistry Environmental Chemistry Additional Questions Solved
I. Choose the correct answer
Question 1.
The type of pollution caused by the spraying of DDT is
(a) air and soil
(b) air and water
(c) air
(d) air, water, and soil
Answer:
(d) air, water, and soil
Question 2.
Which one of the following gases is not present in troposphere?
(a) N2O2
(b) CO2
(c) N2
(d) water vapours
Answer:
(c) N2
Question 3.
The gas responsible for ozone depletion:
(a) NO and freons
(b) SO2
(c) CO2
(d) CO
Answer:
(a) NO and freons
Question 4.
Which one of the following is produced as a result of incomplete combustion of coal’?
(a) CO2
(b) CO
(c) SO2
(d) SO3
Answer:
(b) CO
Question 5.
The main element of smog is
(a) O3 and PAN
(b) O3
(c) PAN
(d) PPN and PBN
Answer:
(a) O3 and PAN
Question 6.
Photochemical smog occurs in warm, dry and sunny climate. One of the following is not among-st the components of photochemical smog. Identify it.
(a) NO2
(b) O3
(c) SO2
(d) Unsaturated hydrocarbons
Answer:
(c) SO2
II. Match the following
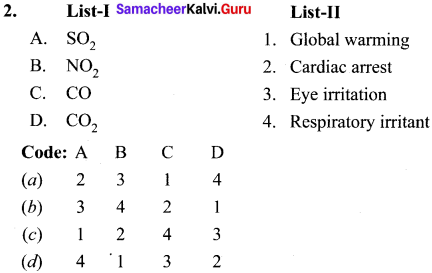
1.
Answer:
![]()
Answer:
![]()

Answer:
![]()
Answer:
![]()

Answer:
![]()
Answer:
![]()
III. Fill in the blanks
Question 1.
An example of a biodegradable pollutant is ………
Answer:
plant waste.
Question 2.
An example of a non-biodegradable pollutant ………
Answer:
metal waste.
Question 3
……… is the lowest layer of the atmosphere.
Answer:
Troposphere.
Question 4.
Ozone is present in ……… layer of the atmosphere.
Answer:
Stratosphere.
Question 5
……… is called the blue planet.
Answer:
Earth.
Question 6.
About 80% of the mass of the atmosphere is present in ………
Answer:
Troposphere.
Question 7.
……… will reduce the oxygen-carrying capacity of the blood.
Answer:
CO
Question 8
……… gas is used in the process of photosynthesis.
Answer:
CO2
Question 9.
……… gas potentially damage plant leaves and retard photosynthesis.
Answer:
NO2
Question 10.
……… is formed by incomplete combustion of coal.
Answer:
CO2
Question 11.
……… binds with haemoglobin and reduce the oxygen-carrying capacity of the blood.
Answer:
CO
Question 12.
……… is carcinogenic and causes irritation in the eyes and mucous membrane.
Answer:
PAH
Question 13.
The earth’s surface gets heated up by a phenomenon called ………
Answer:
Greenhouse effect
Question 14.
The main constituent of layer responsible for global warming is ………
Answer:
CO2
Question 15.
Earth’s average surface temperature would be only about ………
Answer:
-18°C
Question 16.
The pH of rain water is normally ………
Answer:
5.6
Question 17.
……… pair of compounds is present in acid-rain water.
Answer:
HNO3 + H2SO4
Question 18.
……… is an example of viable particulate.
Answer:
Bacteria.
Question 19.
……… is an example of non-viable particulate matter.
Answer:
Cement dust.
Question 20.
Particulate pollutants will result in the health hazard named as ………
Answer:
pneumoconiosis.
Question 21.
Coal miners may suffer from ………
Answer:
Black lung disease.
Question 22.
Textile workers may suifer from ………
Answer:
White lung disease.
Question 23.
………….. aftect childrcns brain. interfers with maturation of RBC’s and even causes cancer.
Answer:
Lead.
Question 24.
……… can be used to reduce particulate pollutant.
Answer:
electrostatic precipitator
Question 25.
……… is the combination of smoke and fog.
Answer:
Smog.
Question 26.
Classical smog called London smog contains ………
Answer:
Coal-smoke and fog.
Question 27.
Classical smog is otherwise called ………
Answer:
reducing smog.
Question 28.
Photochemical smog is otherwise called ………
Answer:
Los angeles smog.
Question 29.
The three main components of photochemical sinog are ………
Answer:
Hydrogen sulphide. dust and PAN.
Question 30.
……… plantation can metabolise nitrogen oxide and control photochemical smog.
Answer:
Pinus tree.
Question 31.
……… acts as an umbrella for the earth and prevent harmful UV radiations.
Answer:
ozone.
Question 32.
……… pair of compounds are found to be highly responsible for depletion of ozone layer.
Answer:
Nitric oxide + CFC
Question 33.
Freons are ………
Answer:
Chiorofluoroalkanes.
Question 34.
Ozone layer is depicted by the reactive ………
Answer:
Chlorine atom.
Question 35.
Examples of water borne diseases are ………
Answer:
Dysentery and cholera.
Question 36.
The standard pH of drinking water is ………
Answer:
6.5 to 8.5
Question 37.
The essential elements for soil are ………
Answer:
N, P, K
Question 38
……… is a better alternative for carcinogenic benzene.
Answer:
Xylene.
Question 39.
The alternate solvent used instead of tetrachloroethylene in dry cleaning is ………
Answer:
Liquefied CO2
Question 40.
……… is used for bleaching clothes in the laundry.
Answer:
H2O2
Question 41.
……… is used to bleach paper.
Answer:
H2O2
Question 42.
……… is the safest pesticide.
Answer:
Neem-based pesticide.
Question 43.
……… acid is most abundant ¡n acid rain.
Answer:
H2SO4
![]()
Question 44.
……… causes less pollution.
Answer:
CO2
Question 45.
Besides CO2 the other greenhouse gas is ………
Answer:
CH4
Question 46.
BOD is a measure of ………
Answer:
Organic pollutant in water.
Question 47.
The pollutant released in the Bhopal gas tragedy was ………
Answer:
Methyl isocyanate.
Question 48.
The greatest affinity for haemoglobin in shown by ………
Answer:
CO
![]()
Question 49.
Eutrophication causes reduction in ………
Answer:
dissolved oxygen.
IV Choose the odd one out
Question 1.
(a) Plant waste
(b) DDT
(c) Plastic
(d) Nuclear waster
Answer:
(a) Plant waste. It is biodegradable pollutant whereas others are non-biodegradable pollutants.
Question 2.
(a) Plant waste
(b) Animal wastes
(c) Paper
(d) Nuclear waste
Answer:
(d) Nuclear waste. It is a non-biodegradable pollutant whereas others are biodegradable wastes.
Question 3.
(a) N2O2
(b) CO2
(c) H2O (Vap)
(d) O3
Answer:
(d) O3 It is present in the stratosphere whereas others are present in the troposphere.
Question 4.
(a) O2+
(b) O+
(c) N2
(d) NO+
Answer:
(c) N2. ills present in mesosphere whereas others are present in the thermosphere.
Question 5.
(a) N2
(b) O2
(c) O3
(d) N2O2
Answer:
(d) N2O2 It is present in the troposphere whereas others are present in the stratosphere.
Question 6.
(a) CH4
(b) CO
(c) CO2
(d) CFC
Answer:
(b) CO. It is a poisonous gas whereas others are responsible for green house effect.
V. Choose the correct pair
Question 1.
(a) CCF : Green house effect
(b) CO : Carcinogenic
(c) PAH : Acid rain
(d) NO : Lung injury
Answer:
(a) CCF : Green house effect
Question 2.
(a) NO2 : Green house effect
(b) CFC : asthma and lung injury
(c) PAH : carcinogenic
(d) CO2 : green house effect
Answer:
(a) NO2 : Green house effect
Question 3.
(a) Classical smog : NO2 and O2
(b) London smog : SO2, SO2 and humidity
(c) Photochemical smog : CO2 and CO
(d) Los Angel smog : NO2 and NO3
Answer:
(b) London smog : SO2 , SO2 and humidity
VI. Choose the incorrect pair
Question 1.
(a) Photochemical smog : NO2 and O2
(b) Classical smog : SO2, SO3 and humidity
(c) Smog : Smoke and fog
(d) Non-viable particulate : Algae, Fungi
Answer:
(d) Non viable particulate: Algae, Fungi
Question 2.
(a) Viable particulate : bacteria, fungi
(b) Non-viable particulate : smoke, dust
(c) Acid rain : HCl + HNO2
(d) Photochemical smog : NO2 + O3
Answer:
(c) Acid rain : HCl + HNO2
Question 3.
(a) Lead : Damage to kidney, liver
(b) Sulphate : Laxative effect
(c) Nitrage : Blue baby syndrome
(d) TDS : Damage to bone and teeth
Answer:
(d) TDS : Damage to bone and teeth
Question 4.
(a) Insecticides : DDT. BCHs
(b) Herbicides : Organo mercury compounds
(c) Herbicides : Sodium chlorate, sodium arsenite
(d) Industrial waste : Mercury. copper
Answer:
(b) Herbicides : Organo mercuiy compounds
VII. Assertion & Reason
Assertion (A) : Depletion of ozone layer causes skin cancer.
Reason (R) : Depletion olozone layer will allow more UV rays to reach the earth surface and cause skin cancer.
(a) Both (A) and (R) are correct and (R)is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (R).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 2.
Assertion (A) : UV radiation damages fish productivity.
Reason (R) : UV radiations affect the growth of phytoplankton as a result food chain in the ocean is disturbed.
(a) Both (A) and (R) are correct hut (R) is not the correct explanation of (A).
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 3.
Assertion (A) : The pH of acid rain is less than 5.6.
Reason (R) : CO, present in the atmosphere dissolves in rain water and forms carbonic acid,
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct hut (R) is not correct explanation of (A).
(c) (A) is correct bitt (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Samacheer Kalvi 11th Chemistry Environmental Chemistry 2 Mark Questions and Answers
Question 1.
What is meant by environmental pollution?
Answer:
Any undesirable change in our environment that has harmful effects on plants, animals, and human beings is called environmental pollution.
Question 2.
Write a note on the constituents of the earth’s atmosphere.
Answer:
Earth’s atmosphere is a layer of gases retained by earth’s gravity. it contains roughly 78% Nitrogen, 21% Oxygen. 0.93% Argon, 0.04% Carbon dioxide. traces of other gases and little amount of water vapour. This mixture is commonly known as air.
Question 3.
What is called the troposphere? How many layers are present in it? Give their names.
Answer:
- Air pollution
- Water pollution
- Soil pollution.
Question 4.
Write about hydrosphere (or) Why Earth is called a Blue planer?
Answer:
- The hydrosphere includes all types of water sources like oceans, seas, rivers, lakes. streams, underground water, polar ice-caps, clouds etc.
- It covers about 75% of the earth’s surface. Hence earth is called a Blue planet.
Question 5.
What is the lithosphere?
Answer:
The lithosphere includes soil, rocks, and mountains which are solid components of Earth.
Question 6.
What is the biosphere?
Answer:
The biosphere includes the lithosphere. hydrosphere and atmosphere integrating the living organisms present in the lithosphere, hydrosphere and atmosphere.
Question 7.
What is air pollution?
Answer:
Air pollution is defined as any undesirable change in air which adversely affects living organisms. Air pollution is limited to the troposphere and stratosphere.
Question 8.
What are the types of air pollutants? Give examples.
Answer:
1. Air pollutants exists in two major forms namely:
(a) Gaseous air pollutants
(b) Particulates
2. Gaseous air pollutants:
Oxides of sulphur, oxides of nitrogen, oxides of carbon and hydrocarbons are gaseous air pollutants.
3. Particulate pollutants:
Particulate pollutants are small solid particles and liquid droplets suspended in air.
Exarnple:
dust, pollen, smoke, soot, aerosols.
Question 9.
Define greenhouse effect.
Answer:
Greenhouse effect is defined as the heating up of the earth’s surface due to trapping of infrared radiations reflected by earth’s surface by the CO2 layer in the atmosphere.
Question 10.
What is Global warming?
Answer:
The heating up of the earth through the greenhouse effect is called Global warming.
Question 11.
When rain water is named as acid rain?
Answer:
Rain water normally has a pH of 5.6 due to dissolution of atmospheric CO2 particles into it. Oxides of sulphur and nitrogen in the atmosphere are absorbed by droplets of water that make up clouds and get chemically converted into sulphuric acid and nitric acid. As a result the pH of rainwater drops below 5.6 and hence it is called acid rain.
Question 12.
What is stone leprosy? How is it formed?
Answer:
- The attack on the marble of buildings by acid rain is called stone leprosy.
- Acid rain causes extensive damage to buildings made up of marble CaCO + H2SO4 CaSO4 + H2O + CO2↑
Question 13.
What are fumes? Give one example.
Answer:
Fumes are one of the non-viable particulate pollutants air. They are obtained by condensation of vapours released during sublimation, distillation, boiling and calcination. For example, organic solvents, metals and metallic oxides form fume particles.
Question 14.
What are the techniques to reduce particulate pollutants?
Answer:
The particulates from air can be removed by using electrostatic precipitators, gravity settling chambers, wet scrubbers or by cyclone collectors. These techniques are base on washing away or settling of the particulate matter.
Question 15.
How will you control photochemical smog?
Answer:
The formation of photochemical smog can be suppressed by preventing the release of nitrogen oxide and hydrocarbons into the atmosphere from motor vehicles by using catalytic convertors in engines. Plantation of certain trees like Pinus, Pyrus Querus vitus and Juniparus can metabolise nitrogen oxide.
Question 16.
What is meant by water pollution’?
Answer:
Water pollution is defined as the addition of foreign substances or factors like heat which degrades the quality of water so that it becomes an health hazard or unfit for use.
Question 17.
What are the sources of water pollution? Give examples.
Answer:
- The water pollutants originate form both natural as well as human activities. The source of water pollution are classified as point and non-point sources.
- Easily identified source of water pollution is called a point source. For example, Municipal and industrial discharge pipes.
- The non-point source cannot be identified easily. For example, agricultural runoffs, mining wastes, acid rain, storm water drainage and construction sediments.
Question 18.
What is BOD’?
Answer:
The total amount of oxygen (in milligrams) consumed by microorganisms in decomposing the waste in one litre of water at 20°C for a period of 5 days is cal Led biochemical oxygen demand (BOD) and its value is expressed in ppm.
Question 19.
What is COD?
Answer:
Chemical oxygen demand (COD) is defined as the amount of oxygen required by the organic matter in a sample of water for its oxidation by a strong oxidising agent like K2Cr2O7 in acidic medium for a period of 2 hours.
Question 20.
What are total dissolved solids (TDS)?
Answer:
- Most of the salts are soluble in water. It includes cations like calcium, magnesium, sodium, potassium, iron and anions like carbonate, bicarbonate, chloride, sulphate, phosphate and nitrate.
- Use of drinking water having TDS (total dissolved solids) concentration higher than 500 ppm causes possibilities of irritation in stomach and intestine.
Question 21.
What are the constituents of soil?
Answer:
Soil is a thin layer of organic and inorganic material that covers the earth’s rocky surface. Soil constitutes the upper crust of the earth, which supports land, plants and animals.
Question 22.
Define – soil pollution.
Answer:
Soil pollution is defined as the build up of persistent toxic compounds, radioactive materials, chemical salts and disease-causing agents in soil which have harmful effects on plant growth and animal health. Soil pollution affects the structure and fertility of soil, ground water quality and food chain in a biological ecosystem.
Question 23.
Explain how industrial waste affects the soil.
Answer:
- Industrial activities have been the biggest contributor to soil pollution especially mining and manufacturing activities.
- Industrial wastes include cyanides. chromates, acids, alkalis and metals like mercury, copper, zinc, cadmium and lead.
- These industrial wastes in the soil surface lies for a long time and make it unsuitable for use.
Question 24.
What is green chemistry?
Answer:
- Efforts to control environment pollution have resulted in the development of chemicals favorable for environment and this branch of science is called green chemistry.
- It is a chemical philosophy encouraging the design of products and processes that reduces or eliminates the use and generation of hazardous substances.
Question 25.
Write a note about the dry cleaning of clothes.
Answer:
Solvents like tetrachloroethylene is used in the dry cleaning of clothes, pollute the ground water and are carcinogenic. In place of tetrachloroethylene liquefied CO2 with suitable detergents an alternate solvent used. Liquefied CO2 is not that harmful for groundwater. Nowadays H2O2 is used for bleaching clothes in laundry that give better results and utilises less water.
Question 26.
Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Answer:
Carbon monoxide gas combines with haemoglobin to form a very stable compound known as carboxy haemoglobin. When its concentration in blood reaches 3-4%, the oxygen carrying capacity of the blood is greatly reduced. This results into headache, nervousness and sometimes death of the person. On the other hand CO2 does not combine with haemoglobin and hence it is less harm ful than CO.
Question 27.
Which gases are responsible for the greenhouse effect? List some of them.
Answer:
CO is mainly responsible for the greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapours, CFCs, and ozone.
Question 28.
A large number of fishes are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish killing.
Answer:
Excessive phytoplankton (organic pollutants such as leaves, grass trash etc.) growth which is present in water is biodegradable. Bacteria decomposes this organic matter in water. During this process when large number of bacteria decomposes the organic matter, they consume the dissolved oxygen in water. When the level of dissolved oxygen falls below 6 ppm then the fishes cannot survive and they die.
Question 29.
How carbon monoxide acts as a poison for human beings’?
Answer:
Carbon monoxide is a poisonous gas because it combines with haemoglobin of RBC to form carboxyhaernoglobin as:
CO + Haemoglobin ⇌ Carboxyhaemoglobin
It inhibits the transport of oxygen to different pans of the body. Thus the body becomes oxygen-starved.
Question 30.
Give three examples in which the principles green chemistry has been applied.
Answer:
- In dry-cleaning, use of liquefied CO2 in place of non-environment friendly tetrachioroethene (Cl2C = CCl2).
- Use of H2O2 in bleaching in place of chlorine.
- In the manufacture of chemicals like ethanal by using environment-friendly chemicals and conditions. ,
Samacheer Kalvi 11th Chemistry Environmental Chemistry 3 Marks Questions and Answers
Question 1.
Write about Bhopal gas tragedy.
Answer:
- On 3rd Decembers. 1984 at Bhopal city in India, by the early morning, an explosion at Union carbide pesticide plant released a cloud of toxic gas (Methyl isocyanate) CH3NCO into the air.
- Since the gas is twice as heavy as air, it did not drift away but formed a blanket over the surrounding area.
- It attacked people’s lungs and affect their breathing staying there or in the nearby areas. Thousands of peopic died and lives of many were ruined. The lungs, brain, eyes, muscles as well as gastrointestinal, neurological and immune system of those people who survived were severely affected.
Question 2.
Explain how the oxides of sulphur pollute the atmospheric air. Give its haririful eflects.
Answer:
- Sulphur dioxide and sulphur trioxide are produced by burning sulphur containing fossil fuels and by roasting of suiphide ores.
- SO2 is a poisonous gas for both animals and plants. SO2 causes eye irritation, coughing and respiratory diseases like asthma, bronchitis.
- SO2 is oxidised to more harmful SO3 gas in the presence of particulate matter present in the polluted air:
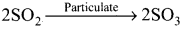
SO2 combines with atmospheric water vapour to form H2SO4 which comes down along with rain in the form of acid rain: - Acid rain causes stone leprosy. affect aquatic ecosystem, corrode water pipes and causes respiratory ailment in humans and animals.
Question 3.
How oxides of nitrogen are harmful?
Answer:
(i) Oxides of nitrogen are produced during high temperature combustion processes, by oxidation of nitrogen in air and it is formed the combustion of fuels such as coal, diesel and petrol.
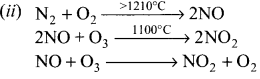
(iii) The oxides of nitrogen are converted into nitric acid which comes down in the form of acid rain. They also form reddish brown haze in heay traffic.
(iv) Nitrogen dioxide potentially damage plant leaves and retard photosynthesis.
(v) NO2 is a respiratory irritant and it can cause asthma and lung injury.
Question 4.
Explain how hydrocarbons pollute the atmospheric air.
Answer:
(i) Microbiological (Pathogens):
Disease causing microorganisms like bacteria, viruses and protozoa are most serious water pollutants. They come from domestic sewage and animal excreta. Fish and shellfish can become contaminated and people who eat them can become ill. Some serious diseases like polio and cholera are water borne diseases. Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis which cause gastrointestinal diseases.
(ii) Organic wastes:
Organic matter such as leaves, grass, trash etc can also pollute water. Water pollution is caused by excessive phytoplankton growth within water. Microorganisms present in water decompose these organic matter and consume dissolved oxygen in water.
Write flotes about great London smog.
Answer:
- The great smog of London (1952) was an instance of severe air pollution that affected the London from 5th December 9th of December, 1952 and then dispersed quickly when the whether changed.
- It causes major disruption by reducing the visibility and even penetrating indoor areas.
- Government medical reports estimated that 4000 people had died as a direct result of smog and 100.000 were made ill by the smog effect on their respiratory tract.
Question 6.
What are the effects of classical smog?
Answer:
- Smog is primarily responsible for acid rain.
- Smog results in poor visibility and it affects air and road transport.
- It also causes bronchial irritation.
Question 7.
Explain how oxides of nitrogen are introduced directly into the stratosphere?
Answer:
- Nitrogen oxides are introduced directly into the stratosphere by the supersonic jet aircraft engines in the form of exhaust gases.
- These oxides arc released by combustion of fossil fuels and nitrogen fertilisers. Inert nitrous oxide in the stratosphere is photochemically converted into more reactive nitric oxide.
- Oxides of nitrogen catalyses the decomposition of ozone and are themselves regenerated. Ozone gets depleted as follows:
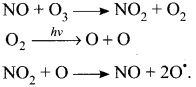
Thus NO is regenerated in the chain reaction.
Question 8.
How chemical wastes pollute water?
Answer:
- A whole variety of chemicals from industries, such as metals and solvents are poisonous to fish and other aquatic life.
- Toxic pesticides can accumulate in fish and shell fish and poison the people who eat them.
- Detergents and oil float spoils the water bodies.
- Acids from mine drainage and salts from various sources can also contaminate water sources.
Question 9.
Explain the presence of fluoride in water and its hazardous effects?
Answer:
- Fluoride ion deficiency in drinking water causes tooth decay.
- The fluoride ions make the enamel on teeth much harder by converting hydroxyapatite
[3(Ca3(PO4)2. Ca(OFl)2]. the enamel on the surface of the teeth into much harder fluorapatite [3(Ca3(PO3)2. CaF2] - Fluoride ion concentration above 2 ppm causes brown mottling of teeth. Excess fluoride causes damage to bones and teeth.
Question 10.
Explain the harmful effects of
- lead
- Nitrate in drinking water.
Answer:
- Drinking water containing lead contamination above 50 ppm can cause damage to liver, kidneys and reproductive system.
- Use of drinking water having concentration of nitrates higher than 45 ppm may cause (blue baby syndrome) methemoglobinemia disease in chiidrens.
Question 11.
Explain how styrene is produced by traditional and greener routes?
Answer:
1. Traditional route:
This method involve two steps-Carcinogenic benzene reacts with ethylene to form ethyl benzene. After that ethyl benzene undergoes dehydrogenation using Fe2O3 / Al2O3 to give styrene.
2. Greener route:
To avoid carcinogenic benzene. greener routes to start with cheaper and
environment friendly xylene.
Question 12.
How acetaldehyde is commercially prepared by green chemistry?
Answer:
Acetaldehyde is commercially prepared by one step oxidation of ethene in the presence of ionic catalyst in aoiieniis medium with 9% yield.
![]()
Question 13.
What do you mean by ozone hole? What are its consequences?
Answer:
Depletion of ozone layer creates some sort of holes in the blanket of ozone which surrounds us in the atmosphere and this is known as ozone hole.
- With the depletion of the ozone layer, UV radiations filters into the troposphere which leads to ageing of skin. cataract, sunburn, skin cancer etc.
- By killing many of the phytoplanktons, it can damage the fish productivity.
- Evaporation rate increases through the surface and stomata of leaves which can decrease the moisture content of the soil.
Question 14.
What is photo chemical smog? What arc its effects? How can it be controlled?
Answer:
It is a kind of smog formed in warm, dry and sunny climate. It is formed when sunlight is absorbed by SO2 oxides of nitrogen and hydrocarbons. It act as an oxidising agent.
Effects of photo chemical smog:
- It produces irritation in eyes and also in respiratory system.
- It can damage many materials such as metals, stones, building materials etc.
- NO2 present in photochemical smog gives it brown colour which reduces the visibility.
- it is harmful to fabrics, crops and ornamental plants.
Control of photochemical smog:
- By using catalytic converters in automobilës.
- By spraying certain compounds into the atmosphere which generate free radicals that can easily combine with the free radicals that initiate the reaction forming toxic compounds of photochemical smog.
- Certain plants such as Pinus, Juniperus, Pyrus could be also helpful in this matter.
Question 15.|
Write a short note on Eutrophication.
Answer:
Eutrophication is a process by which water bodies receive excess nutrients that stimulates excessive plant growth (algae, other plant weeds). This enhanced plant growth in water bodies is called an algae bloom.
The growth of algae in extreme abundance covers the water surface and reduces the oxygen concentration in water. Thus, bloom-infested water inhibits the growth of other living organisms in the water body. This process in which the nutrient-rich water bodies support a dense plant population kills animal life by depriving it of oxygen and results in loss of biodiversity is known as eutrophication.
Samacheer Kalvi 11th Chemistry Environmental Chemistry 5 Marks Questions and Answers
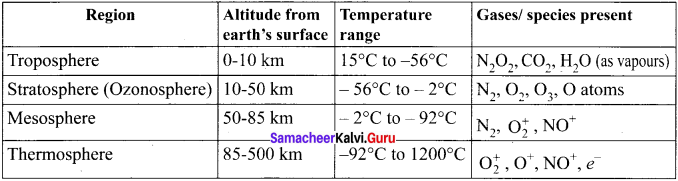
Question 1.
Explain about the different layers of Earth’s atmosphere.
Answer:
Earth’s atmosphere can he divided into different layers with characteristic altitude and temperature.
Question 2.
What arc the health effects of particulate pollutants?
Answer:
1. Dust, mist, fumes etc. are air borne particles which are dangerous for human health. Particulate pollutants bigger than 5 microns are likely to settle in the nasal passage whereas particles of about 10 microns enter the lungs easily and causes scaring or fibrosis of lung lining.
They irritate the lungs and causes cancer and asthma. This disease is called pneumoconiosis. Coal miners may suffer from black lung disease. Textile workers may suffer from white lung disease.
2. Lead particulates affect children’s brain. interferes with the maturation of RBC’s and even causes cancer.
3. Particulates in the atmosphere reduces visibility by scattering and absorption of sun light. It is dangerous for aircraft and motor vehicles.
4. Particulates provide nuclei for cloud formation and increases fog and rain.
5. Particulates deposit on plant leaves and hinder the intake of CO2 from the air and affect photosynthesis.
Question 3.
What are the effects of photochemical smog?
Answer:
- The three main components of photochemical smog are nitrogen oxide, ozone and oxidized hydrocarbons like HCHO, CH2 = CH – CRO and PAN.
- Photochemical smog causes irritation to eyes. skin and lungs. It also increases the chance of asthma.
- high concentration of ozone and NO can cause nose and throat irritation, chest pain, difficulty in breathing.
- PAN is toxic to plants, attack younger leaves and cause bronzing and glazing of their surfaces.
- It causes corrosion of metals, stones, building materials and painted surfaces.
Question 4.
Explain the environmental impacts of ozone depletion.
Answer:
1. The formation and destruction of ozone is a regular natural process, which never disturbs the equilibrium level of ozone in the stratosphere. Any change in the equilibrium level of ozone in the atmosphere will adversely affect the Life in biosphere in the following ways.
2. Depletion of ozone layer will allow more UV rays to reach the earth surface and would cause skin cancer and also decreases the immunity level in human beings.
3. UV radiations atlecis plant proteins which lead to harmful mutation in plant cells.
4. UV radiations affect the growth of phytoplankton and as a result ocean food chain is disturbed and it even damages the fish productivity.
Question 5.
Explain the list of major water pollutants and their sources.
Answer:
- Microorganisms – Domestic sewage, domestic wastewater, dung heap
- Organic wastes – Domestic sewage, animal excreta, food processing factory wastc. detergents and decayed animals and plants
- Plant nutrients – Chemical fertilisers
- Heavy metals – Heavy metal producing factories
- Sediments – Soil erosion by agriculture and strip-mining
- Pesticides – Chemicals used for killing insects, fungi and weeds
- Radioactive substances – Mining of uranium-containing minerals
- Heat – Water used for cooling in industries
Question 6.
What are the major sources that cause soil pollution?
Answer:
Artificial fertilizers:
Soil nutrients are useful for the growth of plants. Plants obtain carbon, hydrogen and oxygen from air or water, whereas other essential nutrients like nitrogen, phosphorous, potassium, calcium, magnesium, sulphur are being absorbed from soil. To remove the deficiency of nutrients in soil, farmers add artificial fertilizers. Increased use of phosphate fertilizers or excessive use of artificial fertilizers like NPK in soil, results in reduced yield in that soil.
Pesticides:
Pesticides are the chemicals that are used to kill or stop the growth of unwanted organisms. But these pesticides can affect the health of human beings.
These are further classified as
Insecticides:
Insecticides like DDT, BHC, aldrin etc. can stay in soil for long period of time and are absorbed by soil. They contaminate root crops like carrot, raddish, etc.
Fungicide:
Organo mercury compounds Eire used as most common fungicide. They dissociate in soil to produce mercury which is highly toxic.
Herbicides:
Herbicides are the chemical compounds used to control unwanted plants. They are otherwise known as weed killers.
Example:
Simple sodium chlorate (NaClO3) and sodium arsenite (Na3AsO3). Most of the herbicides are toxic to mammals.
Industrial wastes:
Industrial activities have been the biggest contributor to soil pollution especially the mining Eind manufacturing activities.
Large number of toxic wastes are released from industries. Industrial wastes include cyanides, chromates, acids, alkalis, and metals like mercury, copper, zinc, cadmium, and lead, etc. These industrial wastes on the soil surface lie for a long time and make them unsuitable for use.
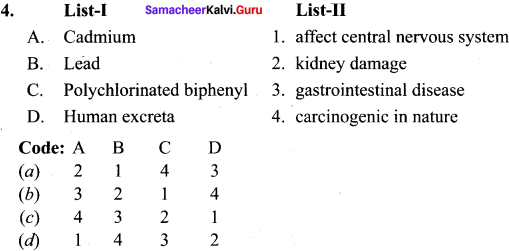
Question 7.
What are the harmful effects of chemical water pollutants?
Answer:
- Cadmium and mercury can cause kidney damage.
- Lead poisoning can lead to severe damage to the kidneys. liver and brain. It also affects the central nervous system.
- Polychlorinated biphenyl causes skin diseases and is carcinogenic in nature.
Question 8.
Explain green chemistry in day-to-day life.
Answer:
1. Dry cleaning of clothes:
Solvents like tetrachloroethylene used in dry cleaning of clothes, pollute the groundwater and are carcinogenic. In place of tetrachloroethylene, liquefied CO2 with suitable detergent is an alternate solvent used. Liquefied CO2 is not harmful to the groundwater. Nowadays H2O2 is used for bleaching clothes in the laundry, gives better results, and utilises less water.
2. Bleaching of paper:
The conventional method of bleaching was done with chlorine. Nowadays H2O2 can be used for bleaching paper in the presence of a catalyst.
3. Synthesis of chemicals:
Acetaldehyde is commercially prepared by one-step oxidation of ethene in the presence of an ionic catalyst in an aqueous medium with 90% yield.
![]()
4. Instead of petrol, methanol is used as a fuel in automobiles.
5. Neem-based pesticides have been synthesized, which are safer than chlorinated hydrocarbons.
We believe the provided Tamilnadu State Board for Class 11th Chemistry Solutions Chapter 15 Environmental Chemistry Guide Pdf Free Download will benefit you. In case, do you have any questions regarding Samacheer Kalvi 11th Chemistry Solutions Book Solutions Chapter 15 Environmental Chemistry Pdf, Questions, and Answers, leave a comment below and we will get back to you at the earliest. Meanwhile, Bookmark our site for more information on State Board Solutions for various subjects.