The Samacheer Kalvi Class 11th Chemistry Solutions Chapter 14 Haloalkanes and Haloarenes Questions and Answers prevailing here are designed by the academic subject expertise in accordance with the state board prescribed syllabus and books. Aspirants who require help in preparing the Tamilnadu State Board Class 11th Chemistry Solutions Chapter 14 Haloalkanes and Haloarenes exercise questions can rely on the Samacheer Kalvi 11th Chemistry Solutions pdf for Chapter 14 Haloalkanes and Haloarenes given in this article.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 14 Haloalkanes and Haloarenes
Students who are excited for Tamilnadu State Board for Class 11th Chemistry Solutions Chapter 14 Haloalkanes and Haloarenes can find detailed and step wise solutions for all questions of Chapter 14 Haloalkanes and Haloarenes from here. Simply tap on the links available over here for which you have to view/download the Samacheer Kalvi Class 11th Solutions for Chemistry Solutions chapter 14 Haloalkanes and Haloarenes Questions and Answers. All these solutions are free to download and easy to access online or offline so that you can prepare well for the exams at any time.
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes Multiple Choice Questions
Question 1.
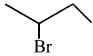
The IUPAC name of 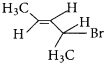 is ………….
is ………….
(a) 2-Bromopent – 3 – ene
(b) 4-Bromopent – 2 – ene
(c) 2-Bromopent – 4 – ene
(d) 4-Bromopent – 1 – ene
Answer:
(b) 4 – Bromopent – 2 – ene
Question 2.
Of the following compounds. which has the highest boiling point’?
(a) n-Butyl chloride
(b) Isobutyl chloride
(c) t-Butyl chloride
(d) n-propyl chloride
Answer:
(a) n-Butyl chloride
Question 3.
Arrange the following compounds in increasing order of their density.
(A) CCl4
(B) CHCl3
(C) CH2Cl2
(D) CH3Cl
(a) D<C<B<A
(b) C<B<A<D
(c) A<B<C<D
(d) C<A<B<D
Answer:
(a) D<C<B<A
Question 4.
With respect to the position of – Cl in the compound CH3 – CH = CH – CH2 – Cl, it is classified as ………..
(a) Vinyl
(b) Allyl
(c) Secondary
(d) Aralkyl
Answer:
(b) Allyl
Question 5.
What should be the correct IUPAC name of diethyl chioromethane?
(a) 3-Chioropentane
(b) 1-Chloropentane
(c) 1-Chloro- 1, 1, diethylmethanc
(d) 1-Chloro- 1 -ethylpropane
Answer:
(a) 3-Chioropentane
Question 6.
C-X bond is strongest in …………
(a) Chioromeihane
(b) lodomethane
(c) Bromomethane
(d) Fluoromethanc
Answer:
(d) Fluoromethane
Question 7.
In the reaction 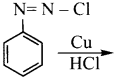 X + N2, X is …………
X + N2, X is …………
Answer:
Question 8.
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH– ion?
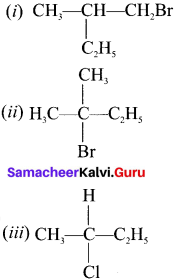
(a) (i)
(b) (ii) and (iii)
(c) (iii)
(d) (i) and (ii)
Answer:
(c) (iii)
Question 9.
The treatment of ethyl formate with excess of RMgX gives –
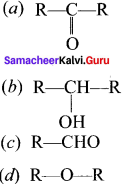
Answer:
(c) R—CHO
Question 10.
Benzene reacts with Cl2 in the presence of FeCl2 and in absence of sunlight to form –
(a) Chiorobenzene
(b) Benzyl chloride
(c) Benzal chloride
(d) Benzene hexachloride
Answer:
(a) Chiorobenzene
Question 11.
The name of C2F4Cl2 is –
(a) Freon – 112
(b) Freon – 113
(c) Freon – 114
(d) Freon – 115
Answer:
(c) Freon – 114
Question 12.
Which of the following reagent is helpful to differentiate ethylene dichloride and ethylidene chloride?
(a) Zn / methanol
(b) KOH / ethanol
(c) Aqueous KOH
(d) ZnCl2 / Cone. HCl
Answer:
(e) Aqueous KOH
Question 13.
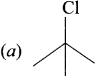
Match the compounds given in Column I with suitable items given in Column II.
Answer:
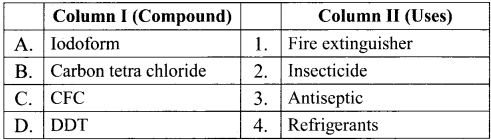
Code:
(a) A→2,B→4,C→1,D→3
(b) A→3,B→2,C→4,D→1
(c) A→1,B→2,C→3,D→4
(d) A→3,B→1,C→4,D→2
Answer:
(d) A→3,B→1,C→4,D→2
Question 14.
Assertion : in monohaloarenes, electrophilic substitution occurs at oriho and para positions.
Reason : Halogen atom is a ring deactivator.
(a) If both assertion and reason arc true and reason is the correct explanation of assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(b) If both assertion and reason are true but reason is not the correct explanation of assertion.
Question 15.
Consider the reaction, CH3CH2CH2Br + NaCN → CH3CH2CH2CN + NaBr This reaction will be the fastest in …………
(a) ethanol
(b) methanol
(c) DMF (N, N’ – dimethyl forrnamide)
(d) water
Answer:
(c) DMF (N, N’ – dimethyl fonuarnide)
Question 16.
Freon-12 is manufactured from tetrachioromethane by …………
(a) Wurtz reaction
(b) Swarts reaction
(c) DMF (N, N’ – dimethyl tòrmamide)
(d) water
Answer:
(c) DMF (N, N’ – dimethyl formarnide)
Question 16.
Frcon-12 is manufactured from tetrachioromethane by …………
(a) Wurtz reaction
(b) Swans reaction
(c) Haloform reaction
(d) Gattennann reaction
Answer:
(b) Swarts reaction
Question 17.
The most easily hydrolysed molecule under SN1 condition is …………
(a) allyl chloride
(b) ethyl chloride
(c) isopropyl chloride
(d) benzyl chloride
Answer:
(a) benzyl chloride
Question 18.
The carbocation formed in SN1 reaction of alkyl halide in the slow step is …………
(a) sp3 hybridised
(b) sp2 hybridised
(c) sp hybridised
(d) none of these
Answer:
(b) sp2 hybridised
Question 19.
The major products obtained when chiorobenzene is nitrated with HNO3 and cone. H2SO4
(a) 1-chloro-4-nitrobenzenc
(b) 1-chloro-2-n itrobenzene
(c) 1-chloro-3-n itroberizene
(d) 1-chloro- 1 -nitrobenzene
Answer:
(a) 1 -chloro-4-nitrobenzene
Question 20.
Which one of the following is most reactive towards nucleophilic substitution reaction?
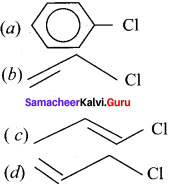
Answer:
![]()
Question 21.
Ethylidene chloride on treatment with aqueous KOH gives …………
(a) acetaldehyde
(b) ehtylene glycol
(c) formaldehyde
(d) glyoxal
Answer:
(a) acetaldehyde
Question 22.
The raw material for Rasching process is …………
(a) chiorobenzene
(b) phenol
(c) benzene
(d) anisole
Answer:
(c) benzene
Question 23.
Chloroform reacts with nitric acid to produce …………
(a) nitro-toluene
(b) nitro-glycerine
(c) chloropicrin
(d) chioropicric acid
Answer:
(c) chioropicrin
Question 24
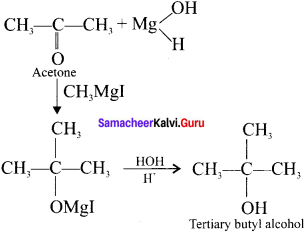
Acetone ![]() X, X is …………
X, X is …………
(a) 2-propanol
(b) 2-methyl-2-propanol
(c) 1 -propanol
(d) acetonol
Answer:
(b) 2-methyl-2-propanol
Question 25.
Silver propionate when refluxed with Bromine in carbon tetrachioride gives …………
(a) propionic acid
(b) chioroethane
(c) bromoethane
(d) chioropropane
Answer:
(c) bromoethane
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes Short Answer Questions
Question 26.
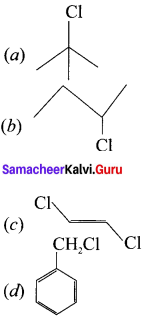
Classify the following compounds in the form of alkyl. allylic, vinyl, benzylic halides:
(a) CH3-CH = CH-Cl
(b) C6H5CH2I
![]()
(d) CH2 = CH – Cl
Answer:
(a) CH3-CH = CH-Cl – Allylic halide
(b) C6H5CH2 – Benzylic halide
![]()
(d) CH2 = CHCl – Vinyl halide
Question 27.
Why chlorination of methane is not possible in dark?
Answer:
- Chlorination of methane is a free radical substitution reaction.
- Before chlorine reacts with methane, the Cl-Cl single bond must break to form free radicals and this can only be done in the presence of ultraviolet light.
- In dark, chlorine free radicals formation is not possible and so chlorination of methane is not possible in dark.
- The ultraviolet light is a source of energy and it being used to break of Cl-Cl and
produce Cl free radical Free radical’s which can attack methane. in dark this is not possible.
Question 28.
How will you prepare n-propyl iodide from n-propyl bromide?
Answer:
Finkeistein reaction:
n-propyl bromide on heating with a concentrated solution of sodium iodide in dry acetone gives n-propyl iodide. This SN2 reaction is called Finkelstein reaction.
![]()
Question 29.
Which alkyl halide from the following pair is –
1. chiral
2. undergoes faster SN2 reaction?

Answer:
1.
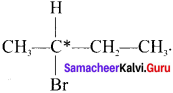 Here the C*is chiral carbon atom and it is surrounded by four different groups. Br
Here the C*is chiral carbon atom and it is surrounded by four different groups. Br
2. ![]() i.e., 1-chiorohutane undergoes faster SN2 reaction. CH3-CH2-CH2Cl is a primary alkyl halide and so it undergoes faster SN2 reaction.
i.e., 1-chiorohutane undergoes faster SN2 reaction. CH3-CH2-CH2Cl is a primary alkyl halide and so it undergoes faster SN2 reaction.
Question 30.
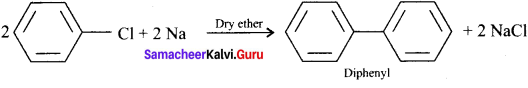
How does chiorobenzene react with sodium in the presence of ether? What is the name of the reaction?
Answer:
Chiorobenzene when heated with sodium in ether solution will forrn biphenyl as the product. This reaction is called fitting reaction.
![]()
Question 31.
Give reasons for polarity of C-X bond in haloalkancs.
Answer:
- Carbon halogen bond is a polar bond as halogens are more electronegative than carbon.
- The carbon atom exhibits a partial positive change (δ+) and halogen atom acquires a partial negative change. (δ–)
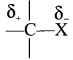
Question 32.
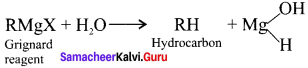
Why is it necessary to avoid even traces of moisture during the use of Grignard reagent?
Answer:
Grignard reagents are highly reactive substances. They react with any source of proton to form hydrocarbons. Even water is sufficiently acidic to convert it into the corresponding hydrocarbon. So it is necessary to avoid even traces of moisture with the Grignard reagent as they arc highly reactive.
Question 33.
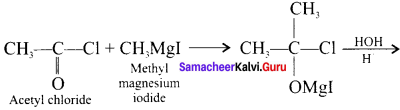
What happens when acetyl chloride is treated with excess of CH3 MgI?
Answer:
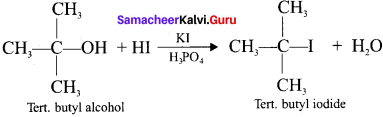
When acetyl chloride is treated with excess of Grignard reagent, the product formed is tertiary butyl alcohol.
Question 34.
Arrange the following alkyl halideïniiicreasing order of bond enthalpy of RX: CH3Br, CH3F, CH3Cl, CH3I
Answer:
increasing order of bond enthalpy of RX is …………
CH3 I bond enthalpy 234 kJ mol-1
CH3 Br bond enthalpy 293 kJ mol-1
CH3 Cl bond enthalpy 351 kJ mol-1
CH3 F bond enthalpy 452 IcI mol-1
CH3 F > CH3 Cl> CH3Br < CH3 I
Question 35.
What happens when chloroform reacts with oxygen in the presence of sunlight?
Answer:
Chloroform undergoes oxidation in the presence of sunlight and air to form phosgene (carboxyl chloride)
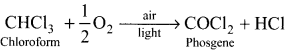
Since phosgene is very poisonous, therefore its presence makes chloroform unfit for use as anaesthetic.
Question 36.
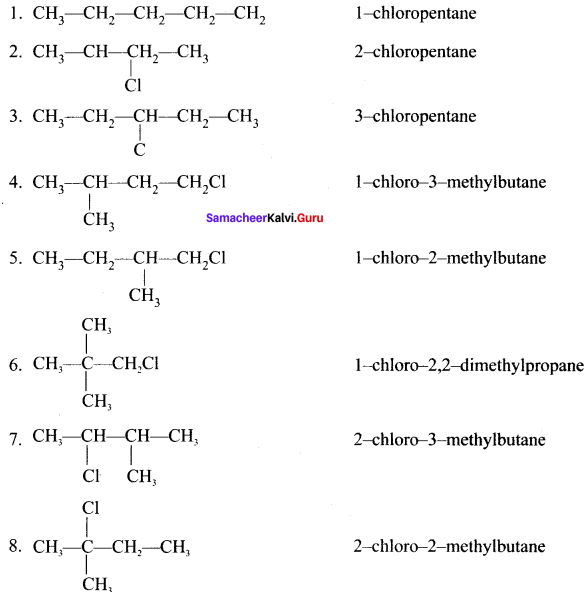
Write down the possible isomers of C5H11Br and give their IUPAC and common names.
Answer:
C5H11Br : 8 isomers are possible.
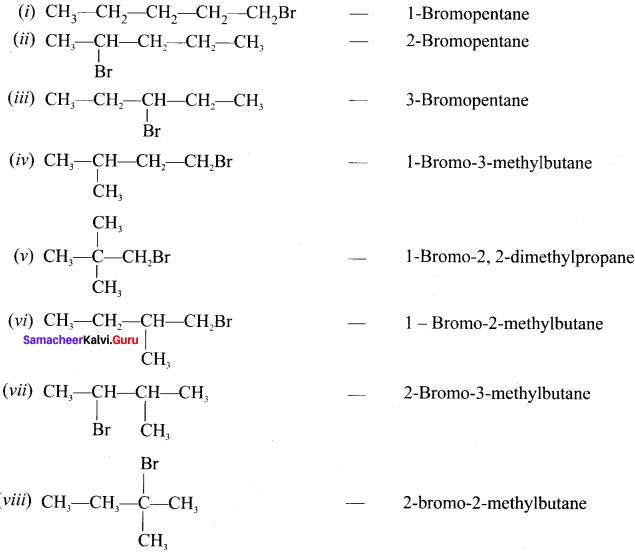
Question 37.
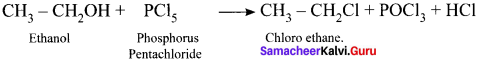
Mention any three methods of preparation of haloalkanes from alcohols.
Answer:
Haloalkanes are prepared from alcohols by treating with –
- HCl
- PCl5
- SO2Cl2
Answer:
1. Reaction of alcohol with hydrogen halide:
![]()
2. Reaction of alcohol with phosphorous halide:
![]()
3. Reaction of alcohol with thionyl chloride:
Question 38.
Compare SN1 and SN2 reaction mechanisms.
Answer:
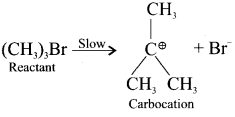
SN1 Mechanism:
1. It is a unimolecular nucleophilic substitution reaction of first order.
2. It takes place in two steps.
3. It leads to racemisation.
4. It mostly take place in tertiary alkyl halides.
5. The rate of the reaction depends only on the concentration one of substrate and so it is a first order reaction.
Step – I
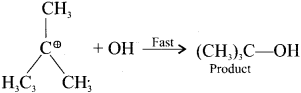
Step – II
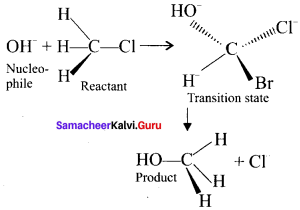
SN2 Mechanism:
1. It is a bimolecular nucleophilic substitution reaction of second order.
2. It takes place in one step.
3. It leads to invertion of configuration.
4. It mostly take place in primary alkyl halides.
5. The rate of the reaction depends on the concentration of both the substrate as well as the nucleophile and so it is a second order reaction.
Question 39.
Compare SN1 and SN2 reaction mechanisms.
Answer:
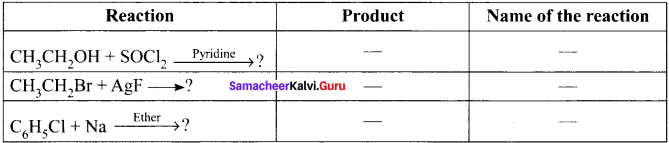
Question 40.
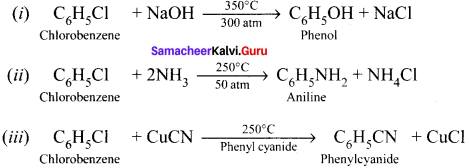
Discuss the aromatic nucicophilic substitution reactions of chiorobenzene.
Answer:
Aromatic nucleophilic substitution reactions:
Dow’s process:
Question 41.
Account for the following –
1. t-butyl chloride reacts with aqueous KOH by SN1 mechanism while n-butyl chloride reacts with SN2 mechanism.
2. p-dichlorobenzene has higher melting point than those of o-and m-dichlorobenzene.
Answer:
1. In t-butyl chloride, there is niore steric hindrance and it involves formation of a stable tertiary carbocation. Therefore it reacts with KOH by SN1 mechanism rather than SN2 mechanism because SN1 mechanism is faourable in case of steric crowding and is directly proportional to partial positive charge on carbon atom. In n-butyl chloride, there is least steric hindrance and involves formation of less stable primary carbocation. Thus it takes place in one step and is favoured by SN2 mechanism.
2. Melting point of p – dichiorobenzene is higher than that of ortho and meta-dichiorobenzene. This is due to the fact that is has a symmetrical structure and therefore, its molecules can easily pack closely in the crystal lattice. p-dichlorobcnzene being more symmetrical fits closely in the crystal lattice and has stronger intermolecular attraction than o & m isomers. So p-isomer has high melting point than the corresponding o & m-isomers.
Question 42.
In an experiment ethyliodide in ether is allowed to stand over magnesium pieces. Magnesium dissolves and product is formed
(a) Name the product and write the equation for the reaction.
(b) Why all the reagents used in the reaction should be dry? Explain.
(c) How is acetone prepared from the product obtained in the experiment?
Answer:
(a) When ethyl iodide in ether is allowed to stand over magnesium pieces. the product formed will be Ethyl magnesium iodide.

(b) Gngnard reagent are highly reactive compounds and react with any source of proton to give hydrocarbons. Even when water is there, it is sufficiently acidic to convert it into the corresponding hydrocarbon. So it is necessary to avoid even traces of moisture with the grignard reagent as they are highly reactive. That is why the all reagents used in the reaction should be dry.

Methyl magnesium iodide reacts with acetyl chloride to form acetone.
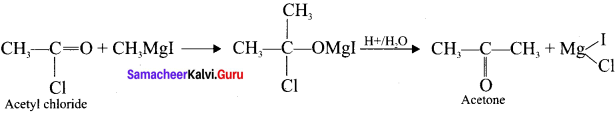
Question 43.
Write a chemical reaction useful to prepare the following:
1. Freon- 12 from carbon tetrachioride.
2. Carbon tetrachioride from carbon disulphide.
Answer:
1. Freon-12 from carbon tetrachioride:
Freon- 12 is prepared by the action of hydrogen fluoride on carbon tetrachioride in the presence of catalytic amount of antimony pentachloride. This is called “swartz reaction”.

2. Carbon tetrachioride from carbon disuiphide:
Carbon disuiphide reacts with chlorine gas in the presence ofanhydrous AlCl3 as catalyst to give carbon tetrachloridc.

Question 44.
What are Freons? Discuss their uses and environmental effects.
Answer:
Freons are the chiorofluoro derivatives of methane and ethane.
Freon is represented as Freon – cba
Where, c = number of carbon atoms, b = number of hydrogen atoms, a = total number of fluorine atoms.
CF2Cl2 = 0
c = 1 – 1 = 0
H = 0 +1 = 1
F = 2
So Freon – 12 is CF2Cl2
Uses of Freons:
- Freons are used as refrigerants in refrigerators and air conditioners.
- it is used as a propellant for aerosols and foams.
- it is used as propellant for foams to spray out deodorants, shaving creams and insecticides.
Environmental effects of Freons:
1. Freon gas is a very powerful greenhouse gas which means that it traps the heat normally radiated from the earth out into the space. This causes the earth’s temperature to increase, resulting in rising sea levels, droughts. stronger storms, flash floods and a host of other very unpleasant effect.
2. As freon moves throughout the air, its chemical ingredients causes depletion of ozone layer. Depletion of ozone increases the amount of ultraviolet radiations that reaches the earths surface, resulting in serious risk to human health. High levels of ozone, in turn, causes resoiratory problems and can also kill olants.
Question 45.
Predict the products when bromoethane is treated with the fòllowing:
1. KNO2
2. AgNO2
Answer:
1. When bromoethane is treated with KNO2 the product formed is Ethyl nitrite.
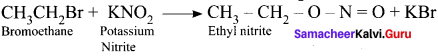
2. When bromoethane is treated with AgNO2 nitroethane will be formed as the product.
![]()
Question 46.
Explain the mechanism of SN1 reaction by highlighting the stereochemistry behind it.
Answer:
1. SN1 stands for unimolecular nucleophilic substitution reaction of first order reaction.
2. The rate of the following SN1 reaction depends upon the concentration of alkyl halide
(RX) and it is independent of the concentration of the nucicophile (OH– ) used.
3. R-Cl + OH– R – OH + Cl–
4. This SN1 reaction follows first order kinetics and it occurs in two steps:
5. SN1 reaction mechanism takes place in tertiary butyl bromide with aqueous KOH a follows:
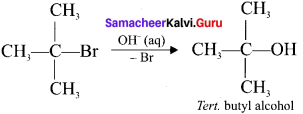
6. the 2 steps of the reaction are:
Step 1:
Formation of carbocation:
The polar C-Br bond breaks first forming a carbocation and bromide ion. This step is slow and hence it is the rate determining step.
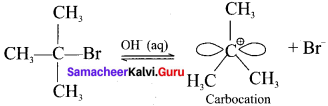
The carbocation has two equivalent lobes of the vaccant 2p orbital, So it can react equally fast form either face.
Step 2:
The nucleophile immediately reacts with the carbocation. This step is fast and hence does not affect the rate of the reaction.
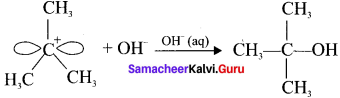
The nucleophile OH can attack carbocation from both the sides.
Question 47.
Write short notes on the the following:
1. Raschig process
2. Dows Process
3. Darzens process
Answer:
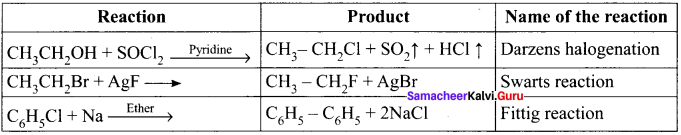
1. Raschig process:
Chiorobenzene is commercially prepared by passing a mixture of benzene vapour in air and HUt over heated cupric chloride.
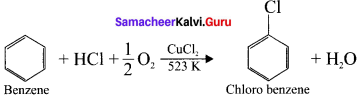
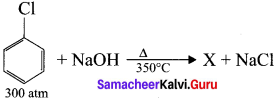
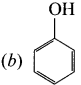
2. Dow’s process:
Chiorobenzene is boiled with Sodium hydroxide to get Phenol. This reaction is called Dow’s process.
![]()
3. Darzens process:
Ethanol reacts with SOCl, in the presence of pyridine to form chloroethane. This reaction is called Darzens process.
![]()
Question 48.
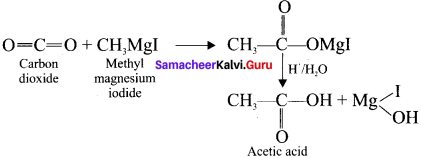
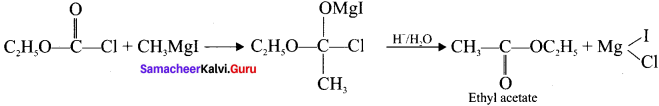
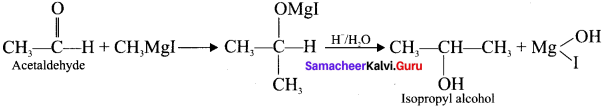
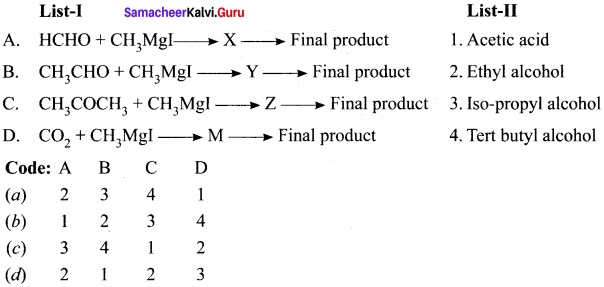
Starting from CH3MgI. How will you prepare the following’?
- Acetic acid
- Acetone
- Ethyl acetate
- Isopropyl alcohol
- Methyl cyanide
Answer:
1. Acetic acid from grignard reagent:
2. Acetone from Gngnard reagent:

3. Ethyl acetate from Grignard reagent:
4. Isopropyl alcohol from Grignard reagent:
5. Methyl cyanide form Grignard reagent:
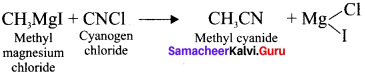
Question 49.
Complete the following reactions:
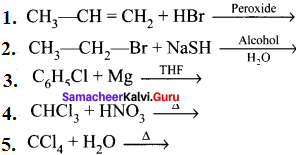
Answer:
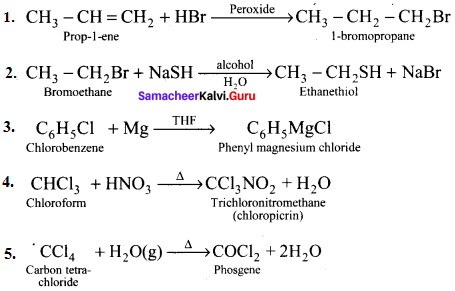
Question 50.
Explain the preparation of the following compounds:
- DDT
- Chloroform
- Biphenyl
- Chioropicrin
- Freon-12
Answer:
1. DUT
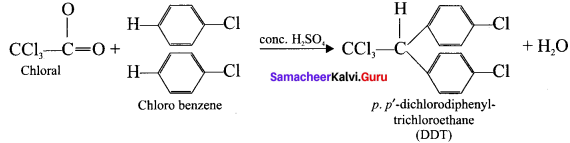
2. Chloroform:
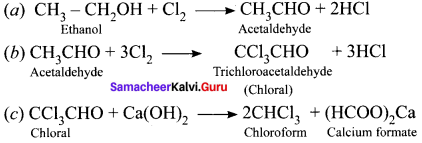
3. Biphenyl:
Fittig reaction:
![]()
4. Chioropicrin.
![]()
5. Freon – 12.
![]()
Question 51.
An organic compound (A) with molecular formula C2H5Cl reacts with KOH gives compounds (B) and with alcoholic KOH gives compound (C). Identify (A), (B) and (C)
Answer:
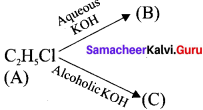
1. An organic compound (A) of molecular formula C2H5Cl is identified as Chioroethane with molecular formula CH2-CH2Cl from the formula.
2. Chioroethane reacts with aqueous KOH to give ethanol, i.e., Aqueous C2H5OH as (B) by substitution reaction.
![]()
3. ChIooethane reacts with alcoholic KOH to give ethene C2H4 as (C) by elimination reaction.
![]()
Question 52.
Simplest alkene (A) reacts with HCl to form compound (B).Compound (B) reacts with ammonia to form compound (C) of molecular formula C2H2N. Compound (C) undergoes carbylarnine test. Identify (A), (B), and (C).
Answer:
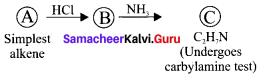
1. The simplest alkene (A) is CH2 = CH2, ethene.
2. Ethene reacts with HCl to give Chioroethane CH2-CH2Cl as (B) by addition reaction.
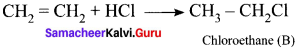
3. Chioroethane reacts with ammonia to give Ethylamine CH3-CH2 NH2 as (C). It is a primary amine and Carbylamine test is the characteristic test for 1° amine.
![]()
Question 53.
A hydrocarbon C3H6 (A) reacts with HBr to form compound (B). Compound (B) reacts with aqueous potassium hydroxide to give (C) of molecular formula C3H8O. What are (A) (B) and (C). Explain the reactions.
Answer:
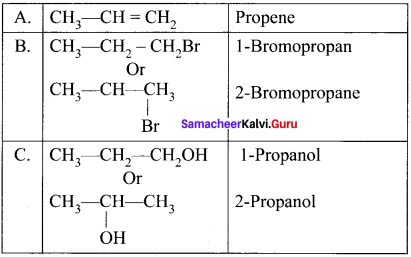
![]()
1. The hydrocarbon with molecular formula C3H6 (A) is identified as propene,
CH3-CH = CH2
2. Propene reacts with HBr to form brornopropane CH3—CH2—CH2Br as (B).
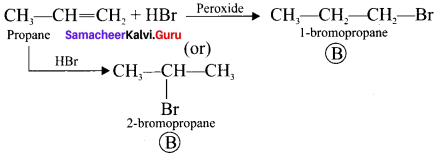
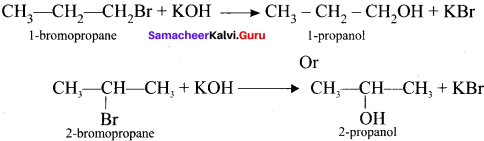
3. 1 -bromopropanc react with aqueous potassium hydroxide to give 1 -propanol
CH3 – CH2 CH2OH as (C).
4. 2-bromo propane reacts with aqueous KOH to give 2-propanol as (C)
Question 54.
Two isomers (A) and (B) have the same molecular formula C2H4Cl2. Compound (A) reacts with aqueous KOK, gives compound (C) of molecular formula C2H4O. Compound (B) reacts with aqueous KOH, gives compound (D) of molecular formula C2H6O2. Identif’ (A), (B), (C) and (D).
Answer:
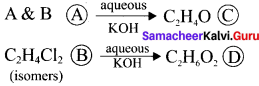
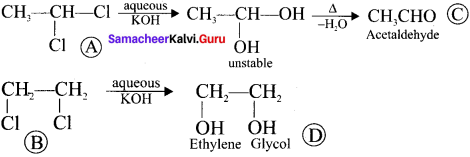
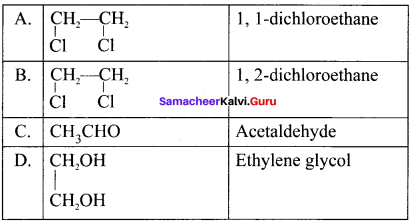
1. The compounds (A) and (B) with the molecular formula C2H4Cl2 are 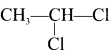 and
and 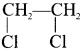 respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.
respectively with IUPAC names: 1, 1-dichloroethane (A) and 1.
2. 1, 1-dichloroethane reacts with aqueous KOH tp give CH3CHO Acetaldehyde as (C).
3. 1 ,2-dichloroethane reacts with aqueous KOH to give ethylene glycol 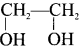 as (D).
as (D).
[Evaluate Yourself ]
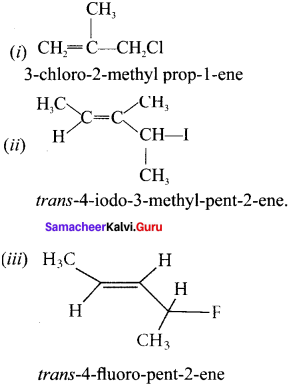
Question 1.
Write the IUPAC name of the following:
Answer:
Question 2.
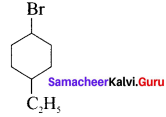
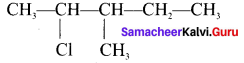
Write the structure of the following compounds:
- 1-Bromo-4-cthylcyclo hexane
- 1 4-Dichlorobut-2-ene
- 2 Chloro-3-methyl pentane
Answer:
1. 1-Bromo-4 ethylcyclo hexane
2. 1. 4-Dichioro but-2-ene
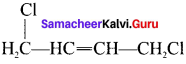
3. 2-Çhloro-3-methyl pentane
Question 3.
Write all possible chain isomers with molecular formula C5H11Cl
Answer:
C5H11Cl:
8 isomers arc possible. These are –
Question 4.
neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly. Justify.
Answer:
1.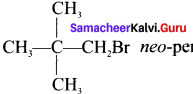 neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.
neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly due to steric hindrance of many alkyl groups. When bromide is attached to neo-pentyl carbon atom, the heterolytic cleavage of C-Br takes place very slowly and substitution is also a very slow reaction.
2. Due to bulky neo-pentyl group, it becomes difficult for a nucleophile to attack from the back side of carbon having C-Br bond.
3. Splitting of C-Br gives a primary carbocation which is very less stable. So neo-pentyl bromide undergoes nucleophilic substitution reactions very slowly.
Question 5.
Why Grignard reagent should be prepared in anhydrous condition’?
Answer:
Grignard reagent are highly reactive species and react with any source of proton to give hydrocarbons. Even when water is there, it is sufficiently acidic to convert it into the corresponding hydrocarbon. So it is necessary to avoid even traces of moisture while preparing grignard reagent as they are highly reactive and that is why gngnard reagent should be prepared in anhydrous conditions.
Question 6.
Haloalkanes undergo nucleophilic substitution reaction whereas haloarenes undergo electrophilic substitution reaction. Comment.
Answer:
Haloalkanes undergo nucleophilic substitution reactions due to high clectronegativity of the halogen atom. The C-X bond in haloalkanes is slightly polar, thereby the C atom acquires a slight positive charge in C-X bond. [lence C atom is a good target for attack for nucleophiles. Therefore C-X atom of haloalkanes is replaced by a nucleophile easily.
![]()
On the other hand in haloarenes, the halogen atom releases electron to the benzene nucleus relatively electron rich with respect to the halogen atom. As a result the electrophilic attack occurs at ortho and para positions. Hence haloarenes undergo electrophilic substitution reactions.
Question 7.
Chloroform is kept with a little ethyl alcohol in a dark coloured bottle. Why?
Answer:
1. Chlorofòrm is slowly oxidised by air in the presence of light to an extremely poisonous gas, carboxyl chloride (phosgene). it is therefore stored in closed dark coloured bottles completely filled so that air is kept out.
2. With the use of 1% ethanol we can stabilise chloroform, because ethanol can convert the poisonous COCl2 gas into non poisonous diethyl carbonate.
COCl2 + 2C2H5OH CO(OC2H5)2 + 2HCl
Question 8.
What is the IUPAC name of the insecticide DDT? Why is their use banned in most of the countries?
Answer:
1. The IUPAC name of the insecticide DDT isp, p-dichloro-diphenyl trichioroethane.
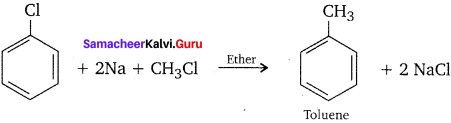
2. Even DDT is an effective insecticide. Now-adays it is banned because of its long term toxic effects.
3. DDT is very persistent in the environment and it has a high affinity for fatty tissues. As a result, DDT gets accumulated in animal tissue fat, in particular that of birds of prey with subsequent thinning of their eggs shells and impacting their rate of reproduction. That is why DDT is banned in most of the countries.
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes Additional Questions Solved
I. Choose The Correct Answer.
Question 1.
Which of the following is an example for polyhalo compounds?
(a) Vnyl iodide
(b) Chiorobenzene
(c) Allyl chloride
(d) Chloroform
Answer:
(d) Chloroform
Question 2.
Which of the following is a secondary haloalkane?
(a) Bromoethane
(b) 2-Chioropropane
(c) 2-Jodo-2-methylpropane
(d) 1-Chloropropane
Answer:
(b) 2-Chioropropane
Question 3.
How many isomers are possible for the formula C4H9Cl?
(a) 3
(b) 2
(c) 4
(d) 5
Answer:
(c) 4
Question 4.
How many isomers are possible for the formula C5H11Br?
(a) 11
(b) 8
(c) 4
(d) 5
Answer:
(b) 8
Question 5.
Which of the following is called Lucas reagent?
(a) Conc. H2SO4 + Anhydrous CuSO4
(b) Conc.HCl + Anhydrous ZnCl2
(c) Dil.HCl + AlCl3
(d) Conc.HCl + ConcHNO2
Answer:
(b) Conc.HCl + Anhydrous ZnCl2
Question 6.
Which of the following mechanism is followed in the halogenation of alkanes in the presence of U-V light?
(a) Nucleophilic substitution
(b) Electrophilic addition
(c) Free radical substitution
(d) Elimination reaction
Answer:
(c) Free radical substitution
Question 7.
The reactivity of alcohols with haloacid is –
(a) 3° > 2° > 1°
(b) 1° > 2° > 3°
(c) 2° > 3° > 1°
(d) 3° > l° > 2°
Answer:
(a) 3° > 2°> 1°
Question 8.
Which of the following reagent is not used to convert alcohol to haloalkane?
(a) H-X
(b) PX5
(c) CCl4
(d) SOCl2
Answer:
(c) CCl4
Question 9.
What is the name of the reaction in which bromoethane is converted to iodoethane by reacting with NaI in acetone?
(a) Hunsdicker reaction
(b) Dow’s process
(c) Finkelstein reaction
(d) Swarts reaction
Answer:
(c) Finkeistein reaction
Question 10.
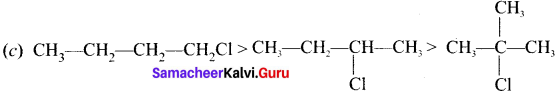
Identify the correct order of boiling point of haloalkanes?
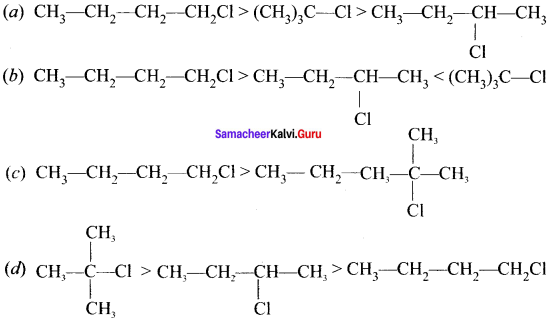
Answer:
Question 11.
Which of the following pair functional groups represents ambident nucleophiles?
(a)-SH-&-OH
(b)-CN-&-NO2
(c)-Br-&-Cl
(d)-O-&-CHO
Answer:
(b)-CN & NO2
Question 12.
Which one following mechanism will be followed when Tartiary butyl chloride is treated with alcoholic KOH?
(a) SN1 mechanism
(b) E1 mechanism
(c) SN2 mechanism
(d) E2 mechanism
Answer:
(b) E1 mechanism
Question 13.
Which one of the following is used for producing pesticìdes?
(a) CHI3
(b) CHCl3
(c) CCl3 NO2
(d) CCl4
Answer:
(b) CHCl3
Question 14.
Which one of the following react with gringnard reagent followed by hydrolysis will yield primary alcohol?
(a) CH3 CHO
(b) HCHO
(c) CH3 COCH3
(d) CO2
Answer:
(b) HCHO
Question 15.
Which one of the following reacts with CH3MgI followed by hydrolysis and gives isopropyl alcohol?
(a) CH3 COCH3
(b) CH3 CHO
(c) HCHO
(d) CNCl
Answer:
(b) CH3 CHO
Question 16.
Which one of the following reacts with CH3 Mg I followed by hydrolysis to yield tert. butyl alchol ?
(a) CH3CHO
(b) HCHO
(c) CH3COOC2H5
(d) CH3COCH3
Answer:
(d) CH3COCH3
Question 17.
Which one of the following reacts with CH3 Mg I followed by acid hydrolysis to yield acetic acid ?
(a) CNCl
(b) CH3COOC2H5
(c) HCOOC2H5
(d) CO2
Answer:
(d) CO2
Question 18.
Which one of the following reagent react with methyl magnesium iodide followed by acid hydrolysis to give ethyl acetate?
(a) Chiorodimethyl ether
(b) Ethyl chloroformate
(c) Ethyl formate
(d) Acetaldehyde
Answer:
(b) Ethyl chioroformate
Question 19.
Which one of the following is used as fibre-swelling agent in textile processing?
(a) Chiorohenzene
(b) Chloroform
(c) Chlorai
(d) Chloroethane
Answer:
(a) Chiorobenzene
Question 20.
Which one of the fillowing is a gemdihalide?
(a) CH3CHCl2
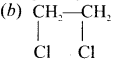
(c) CH3-CH2Cl
(d) C6H4Cl2
Answer:
(a) CH3CHCl2
Question 21.
Which of the following reagent is used to distinguish gem-dihalides and vicinal dihalides?
(a) Alcoholic KOH
(b) Aqueous KOH
(c) FeCl3 / Cl2
(d) Ethanol
Answer:
(b) Aqueous KOH
Question 22.
Which one of the following is used in the conversion of ethyliden dichioride to Acetylene?
(a) Zn + Methanol
(b) KOH + Ethanol
(c) Aqueous NaOH
(d) Alcoholic KOH
Answer:
(b) KOH + Ethanol
Question 23.
Which one of the following is used as a metal cleaning solvent?
(a) Isopropylidene chloride
(b) Methylene chloride
(c) Chloroform
(d) lodoform
Answer:
(b) Methylene chloride
Question 24.
Which one of the following is used as an insecticide and as a soil sterilising agent?
(a) Chloroform
(b) Chlorai
(c) Chioropicrin
(d) Tetrachioromethane
Answer;
(c) Chloropicrin
Question 25.
Which one of the following is used to test primary amincs?
(a) Schiff’s test
(b) Carbylarnine test
(c) Dye test
(d) Silver mirror test
Answer:
(b) Carbyianiine test
Question 26.
Which one of the following is used as propellant for aerosols and foams?
(a) Freons
(b) Methylidene chloride
(c) Chlorai
(d) Chloroform
Answer:
(a) Freons
Question 27.
the product X is –
Question 28.
Which one of the following will undergo SN1 reaction faster?
Answer:
Question 29.
Which one of the following compounds does not undergo nucleophilic substitution reactions at all?
(a) Ethyl bromide
(b) Vinyl chloride
(c) Benzyl chloride
(d) isopropyl chloride
Answer:
(b) Vinyl chloride
II. Match the following.
Question 1.
Answer:
![]()
Question 2.
Answer:
![]()
Question 3.
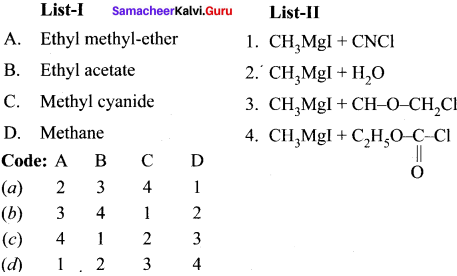
Answer:
![]()
Question 4.
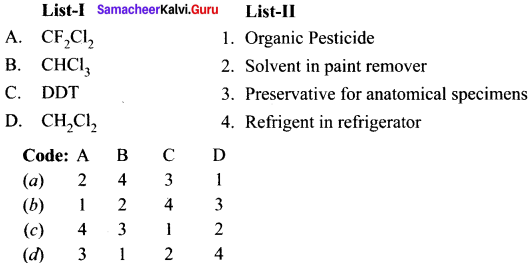
Answer:
![]()
Question 5.

Answer:
![]() “17” />
“17” />
Question 6.
Answer:
![]()
III. Fill in the blanks.
Question 1.
is used in the treatment of typhoid ……….
Answer:
chloramphenicol
Question 2.
………. is used in the treatment of malaria
Answer:
Chioroquine
Question 3.
………. is used as an anesthetic ……….
Answer:
Halothane
Question 4.
………. is used for cleaning electronic equipments ……….
Answer:
Trichioroethylene
Question 5.
The IUPAC name of CH2=CH-CH2Cl is ……….
Answer:
3-Chioro-prop- 1-ene
Question 6.
The structure of Vinyl iodide is ……….
Answer:
CH2=CHI
Question 7.
2-iodo-2-methylpropane belongs to type ……….
Answer:
3°haloalkanes
Question 8.
The structure of 2-Iodo-2-methylpropanc is ………
Answer:
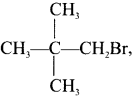
Question 9.
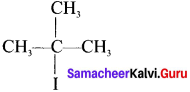
the IUPAC name of this compound is ……..
Answer:
1-Bromo-2, 2-dimethyipropane
Question 10.
The IUPAC name of CH2=CHCl is ………
Answer:
Chioroethene
Question 11.
The IUPAC name of 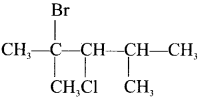 is ………
is ………
Answer:
2-Bromo-3-chloro-2, 4-dimethylpentane.
Question 12.
The IUPAC name of 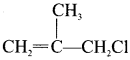 is ………
is ………
Answer:
3-Chloro-2-methyl-prop- 1 -ene
Question 13.
The reactivity of haloacids (HCl , HBr, HI) with alcohol is in the order ………
Answer:
HI > HBr > HCl
Question 14.
The decreasing order of bond length among alkyl halides (CH2I , CH2 Br , CH3F , CH3Cl ) is in the order ………
Answer:
CH3F < CH3Cl < CH3Br < CH3I
Question 15.
The bond strength of C-X for the C-Cl , C-Br, C-I , C-F decreases in the order is ………
Answer:
C-F < C-Cl<C-Br < C-I
Question 16.
The catalyst used in Darzen halogenation of alcohol is ………
Answer:
Pyridine
Question 17.
In Finkeistein reaction, the mechanism followed is
Answer:
SN2
Question 18.
Silver salt of fatty acid is converted to bromo alkanc by ………
Answer:
Hunsdicker reaction
Question 19.
In Swarts reaction, chioroalkane is converted to ………
Answer:
Fluoroalkane.
Question 20.
The conversion of bromoalkane to fluroalkane by heating with AgF is called ………
Answer:
Swartz reaction
Question 21.
The decreasing order of boiling point of haloalkanes CH3 Br , CH3Cl , CH3F CH3I is ………
Answer:
CH3I > CH3Br > CH3CI > CH3F
Question 22.
The correct increasing order of boiling point of haloalkanes CH3Cl , CHCl3 , CH2Cl2 , CCl4 is ………
Answer:
CCl4 > CHCl3 > CH2Cl2 > CH3Cl
Question 23.
Haloalkanes reacts with aqueous solution of KOH to form ………
Answer:
Alcohol
Question 24.
Haloalkane reacts with alcoholic solution of KOH to form ………
Answer:
Alkene
Question 25.
Ethyl bromide reacts with alcoholic AgCN to form ………
Answer:
CH3CH2NC
Question 26.
Ethyl bromide reacts with alcoholic KNO2 to form ………
Answer:
Ethyl nitrite
Question 27.
The reaction in which sodium alkoxide react with haloalkane to form ether in called ………
Answer:
Williamson’s synthesis
Question 28.
Primary alkyl halide react with aqueous NaOH follows ………
Answer:
SN2
Question 29.
Tertiary butyl bromide reacts with aqueous KOH follows ………
Answer:
SN2 mechanism
Question 30.
Ethyl bromide reacts with alcoholic KOH following mechanism. ………
Answer:
E1
Question 31.
The product formed when tertiary butyl chloride is treated with alcoholic KOH is ………
Answer:
Iso-butylene
Question 32.
When 2-bromobutane react with alcoholic KOH, the products formed are ………
Answer;
1-butene & 2-butene
Question 33.
The product formed when iodoethane is treated with HI in the presence of red phosphorous is ………
Answer:
CH3-CH3
Question 34.
……… is used as an antiseptic
Answer:
Iodoform
Question 35.
……… is used for extinguishing the fire caused by oil or petrol under the commercial name pyrene
Answer:
Tetrachioromethane
Question 36.
……… Ethyl formate reacts with methyl magnesium iodide followed by acid hydrolysis to yield
Answer:
Acetaldehyde
Question 37.
……… Ethyl-methyl ether ethene is obtained by the action of methyl magnesium iodide with
Answer:
Chioro-aimethyl-ether
Question 38.
The hybridised state of carbon in haloarenes is ………
Answer:
sp2
Question 39.
The catalyst used in the preparation of chiorobenzene from benzene is ………
Answer:
FeCl3
Question 40.
In the Gattermann reaction of preparation of chiorobenzene from benzene, the catalyst used is ………
Answer:
Cu / HCl
Question 41.
The conversion of benzene diazoniurn chloride to chiorobenzene in the presence of Cu2Cl2 + HCl is named as ………
Answer:
Sandmeyer reaction
Question 42.
Fluorobenzene is prepared from benzene diazonium chloride by ………
Answer:
BaIz-Scheimann reaction
Question 43.
Conversion of benzene to chiorobenzene in the presence of CuCl2 / HCl is named as ………..
Answer:
Gattermann reaction
Question 44.
The conversion of chiorobenzene to phenol by the action of NaOH is called ………..
Answer:
Dow’s process
Question 45.
In Wurtz fittig reaction, chiorohenzene is converted to by reacting it with ethyl chloride.
Answer:
Ethylbenzene
![]()
Question 46.
The product obtained in fittig reaction of chiorobenzene is ………..
Answer:
Biphenyl
Question 47.
The reagent used in the conversion of Chiorobenzene to Benzene is ………..
Answer:
Ni-Al/NaOH
Question 48.
The catalyst used in the preparation of Phenyl magnesium chloride from chiorobenzene is ………..
Answer:
THF
Question 49.
Iso-propylidene chloride is an example of ………..
Answer:
vicinal dihalide
Question 50.
The IUPAC name of 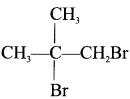 is ………..
is ………..
Answer:
1, 2-dibromo-2-methylpropane
Question 51.
The reagent used in the conversion of ethylene dichioride is ………..
Answer:
Zn + CH3OH
Question 52.
Chloroform is converted to methylene-chioride by the action of ………..
Answer:
Zn + HCl and H2 / Ni
Question 53.
The reagents used in the preparation of chloroform are ………..
Answer:
Ethanol + Bleaching powder
Question 54.
The formula of Chioropicrin is ………..
Answer:
CCl3NO2
Question 55.
The product formed when methylamine react with chloroform and alkali is ………..
Answer:
CH3NC
Question 56.
The product formed when CCI4 reacts with hot water vapours is
Answer:
COCl2
Question 57.
The formula of Freon -11 is ………..
Answer:
CFCl3
Question 58.
The formula of Freon – 12 is ………..
Answer:
CF2Cl2
Question 59.
The catalyst used in the preparation of CCl2F2 from CCl4 and HF is ………..
Answer:
SbCl5
Question 60.
The reagents used to prepare DDT are ………..
Answer:
Chlorai and Chiorobenzene
Question 61.
……….. is used to kill various insects like housefly and mosquitoes.
Answer:
(c) DDT
Question 62.
The name of CFCl3 is
Answer:
Freon-11
Question 63.
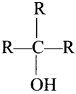
The treatment of acetone with excess of RMgX gives:
Answer:
Question 64.
The most easily hydrolysed molecule under SN2 reaction is………..
Answer:
Ten. butyl chloride
Question 65.
![]() . The product ‘X’ is ………..
. The product ‘X’ is ………..
Answer:
Ethanol
Question 66.
![]() The product X is ………..
The product X is ………..
Answer:
CH4
Question 67.
On heatrng CHCl3 with aqueous NaOH solution, the product formed is
Answer:
HCOONa
Question 68.
Depletion of ozone layer is caused by
Answer:
Freons
Question 69.
Chloropicrin is used as
Answer:
soil sterilizing agent
Question 70.
lodoform can be used as
Answer:
Antiseptic.
Question 71.
in oil fire extinguisher, the compound used pyrene is chemically
Answer:
CCl4
Question 72.
Reaction of ethyl chloride with sodium metal leads to the formation of
Answer:
n-Butane
Question 73.
When chloroform is treated with primary amine and KOH, we get ………..
Answer:
Offensive odours
IV. Choose the odd one out.
Question 1.
(a) CH3Br
(b) CH3-CH2Br
(c) (CH3)3C-Br
(d) CH3-CH2-CH2Br
Answer:
(c) (CH3)3C-Br. It is a haloalkane whereas others are 1° haloalkanes.
Question 2.
(a) Finkeistein reaction
(b) Wurtz reaction
(c) Swarts reaction
(d) Friedel crafts alkylation
Answer:
(d) Friedel crafts alkylation. It is used to prepare aromatic hydrocarbon whereas others are used to prepare alkanes.
Question 3.
(a) PCl5
(b) SOCl2
(c) HCl
(d) HF
Answer:
(cl) HF It is not used to prepare directly fluoro alkane whereas others are used to prepare directly chioro alkanes.
Question 4.
(a) Aerosol spray propellant
(b) Metal cleaning agent
(c) Anaesthetic agent
(d) Solvent in paint remover
Answer:
(c) Anaesthetic agent. It is not the use of methylene chloride whereas others are uses of methylene chloride.
V. Choose the correct pair.
Question 1.
(a) Chiorobenzene + chloral : DDT
(b) Chloroform + HNO3 : Phosgene
(c) Chloroform + Zn / HCl : Methyl isocyanide
(d) Methane + 4Cl2 : Carbon tetra chloride
Answer:
(c) Chlorobenzene + chloral : DDT
Question 2.
(a) Chloroform : Analgesic
(b) Freon : Propellant
(c) Chioropicrin : Antiseptic
(d) DDT : Soil sterilizing agent
Answer:
(b) Freon : Propellant
Question 3.
(a) Freon : Refrigerant
(b) DDT : Antiseptic
(c) Methylene : Soil sterilizing agent
(d) Iodotorni : Anaesthetic
Answer:
(a) Freon : Refrigerant
Question 4.
(a) HCOH + CH3MgI : Secondary alcohol
(b) CH3CHO + CH3MgI : Tertiary alcohol
(c) CH3COCH3 + CH3MgI : Primary alcohol
(d) CO2 + CH3MgI : Acetic acid
Answer:
(d) CO2 + CH3MgI : Acetic acid
Question 5.
(a) (C3H)3C-Cl + alcoholic KOH : SN1 reaction
(b) (CH3)3C-Cl + alcoholic KOH : E1 reaction
(c) (CH3)3C-Cl + aqueous KOH : SN2 reaction
(cl) CH3-Cl + aqueous KOH : SN1 reaction
Answer:
(b) (CH3)3C-Cl + alcoholic KOH : E1 reaction
VI. Choose the incorrect pair.
Question 1.
Answer:
![]()
Question 2.
(a) CH3I > CH3Br > CH3Cl > CH3F : decreasing order of boiling point
(b) CCl4 > CHCl3> CH2Cl2 > CH3Cl : increasing order of boiling point
(c) CH3-CH2-CH2Cl <CH3 CH2 Cl <CH3Cl : increasing order of boiling point
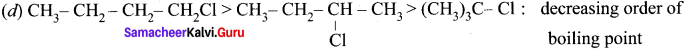
Answer:
(c) CH3-CH2-CH2Cl <CH3 CH2Cl <CH3Cl : increasing order of boiling point
Question 3.
(a) (CH3)3 C-Br + aqueous KOH : SN1 reaction
(b) (CH3)3 C-Br + alcoholic KOH: E1 reaction
(c) CH3Br – aqueous KOH : E2 reaction
(d) CH3Br + aqueous KOH : SN2 reaction
Answer:
(c) CH3Br + aqueous KOH : E2 reaction
Question 4.
(a) 1-chioro propane + Alcoholic KOH : Propene
(b) Tert.butyl bromide + Alcoholic KOH : Isobutylenc
(c) CH3-CH2I + HI + Red p : Ethanc
(d) CH3CHO + CH3 Mg I : Ten. Butyl alcohol
Answer:
(d) CH3CHO + CH3 Mg I : Ten. Butyl alcohol
Question 5.
(a) HCHO + CH3MgI : Primary alcohol
(b) CH3CHO + CH3MgI : Secondary alcohol
(c) CH3COCH3 + CH3MgI : Aromatic alcohol
(d)CO2 + CH3MgI : Acetic acid
Answer:
(c) CH3COCH3 + CH3MgI : Aromatic alcohol
VII. Assertion & Reason.
Question 1.
Assertion (A): The C-I in CH3X is weak.
Reason (R): Larger the size, greater is the bond length and weaker is the bond formed.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(a) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 2.
Assertion (A): Haloalkanes have higher boiling point and melting point than the parent alkanes having the same number of carbon.
Reason (R): The intermolecular forces of attraction and dipole-dipole interactions are
stronger in haloalkanes.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 3.
Assertion (A) : Among isomenc halides, the boiling point decreases with increase in branching in alkyl group.
Reason (R) : With the increase in branching, the molecule attains spherical shape with less
surface area and less forces of interaction.
(a) Both (A) and (R) are correct and (R) is not the correct explanation of (A).
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 4.
Assertion (A): The melting point of para halóbenzene is higher than that of ortho and meta isomers.
Reason (R): The higher melting point of p-isomer is due to its symmetry which leads to more close packing of its molecules in the crystal and subsequently p-isomer have strong intermolecular attractive forces.
(a) both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) both (A) and (R) are correct and (R) is the correct explanation of(A).
Question 5.
Assertion (A) : Haloarenes are insoluble in water.
Reason (R) : Haloarenes are able to form hydrogen bonds with water.
(a) Both (A) and (R) are correct but (R) is the correct explanation of (A).
(b) both (A) and (R) are correct and (R) is not the correct explanation of (A).
(e) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct
Answer:
(c) (A) is correct but (R) is wrong.
Question 6.
Assertion (A) : Haloarenes do not undergo nucleophilic substitution reactions readily.
Reason (R) : The C-X bond in aryl halides is short and stronger and also the aromatic ring is a center of high electron density.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(h) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 7.
Assertion (A): Chloroform vapours can be used as an anaesthetic.
Reason (R): Chloroform vapours depresses the central nervous system and cause unconsciousness.
(a) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 8.
Assertion (A): Nowadays chloroform is not used as an anaesthetic.
Reason (R): Chloroform undergoes oxidation in the presence of light and air to form highly poisonous phosgene.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(cl) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 9.
Assertion (A): DDT is banned now-a-days.
Reason (R): DDT has a long term toxic effect.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
VIII. Choose the correct statement.
Question 1.
(a) Halo alkanes have higher boiling point than the parent alkane with same number of carbons because of strong inter molecular forces of attraction.
(b) The boiling point of halo alkanes decreases with the increase of halogen atoms.
(c) The boiling point of mono halo alkanes decreases with the increase in the number of carbon atoms.
(d) Halo alkanes are soluble in water.
Answer:
(a) Halo alkanes have higher boiling point than the parent alkane with same number of carbons because of strong inter molecular forces of attraction.
Question 2.
(a) Halo alkanes are soluble in water.
(b) The boiling point of halo alkanes increase with the increase in the number of halogen atoms.
(c) The melting point of mono halo alkane decrease with the increase in the number of carbon atoms.
(d) The density of alkyl halides are lesser than those of hydrocarbons of comparable molecular weight.
Answer:
(b) The boiling point of halo alkanes increase with the increase in the number of halogen atoms.
Question 3.
(a) Williamson’s synthesis of ether is an example of nucleophilic substitution reaction.
(b) Reaction of methyl bromide with aqueous potassium hydroxide is an example ofelimination reaction.
(c) Reaction of Tertiary butyl bromide with alcoholic KOH is an example of SN2 reaction.
(d) Reaction of Tertiary butyl bromide with alcoholic KOH is an example of E, reaction.
Answer:
(a) Williamson’s synthesis of ether is an example of nucleophilic substitution reaction.
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes 2 Marks Questions and Answers
Question 1.
Write the IUPAC names of –
- CH2=CHCI
- CH2=CH-CH2Br.
Answer:
- CH2=CHCl : Chloroethene
- CH2=CH-CH2Br : 3-Bromo-prop- 1-ene
Question 2.
Write the structural formula of the following compounds:
- 2-Chloro-2-Methylpropane
- 1 -Bromo-2, 2-Dimethylpropane
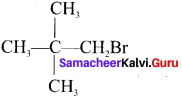
Answer:
1. 2-Chloro-2-Methylpropane
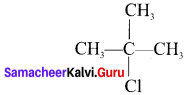
2. 1 -Brorno-2, 2-dimethyipropane
Question 3.
How many isomers are possible for the formula C3H7F? Give their structures and names.
Answer:
C3H7F – 2 isomers are possible.
1. CH3-CH2-CH2F – 1- fiuoropropene
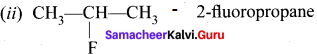
Question 4.
Write the isomeric structures and names for the formula C2H4Cl2.
Answer:
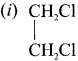 Ethylene dichioride (or) 1, 2-dichioroethane.
Ethylene dichioride (or) 1, 2-dichioroethane.
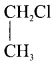 Ethylidene chloride (or) 2-dichioroethane.
Ethylidene chloride (or) 2-dichioroethane.
Question 5.
Draw the structures of –
- 1-brorno-2, 3-dichiorobutane
- 2-bromo-3-chloro-2, 4-dimethyl pentane
Answer:
1. 1 -bromo-2, 3-dichiorobutane
2. 2-bromo-3-chloro-2, 4-dimethy 1 pentane
Question 6.
What happens when HI reacts with ten. butyl alcohol?
Answer:
Question 7.
Explain the action of
(i) PCl5
(ii) PCl3 with ethanol.
Answer:
![]()
![]()
Question 8.
How does HBr react with propene?
Answer:
Propene react with HBr and follows Markovnikov’s rule.

Question 9.
How methane reacts with Cl2 in the presence of light?
Answer:
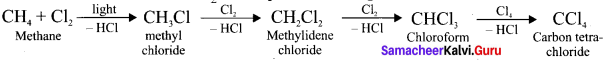
Question 10.
Explain-Finkelstein reaction.
Answer:
Chioro (or) bromoalkane on heating with sodium iodide in dry acetone gives jodo alkane. This reaction is called Finkeistein reaction.
Answer:
![]()
Question 11.
Explain Swans reaction.
Answer:
Chioro (or) bromoalkanes on heating with AgF give fluoroalkanes. This reaction is called Swans reaction.
![]()
Question 12.
What happens when silver propionate reacts with Br, in CCl4?
Answer:
Silver salt of fatty acids (CH3CH2COOAg), e.g., silver propionate treated with Br2 / CCl4 gives bromoalkane. This reaction is called Hunsdicker reaction.
![]()
Question 13.
Haloalkanes have higher boiling point and melting point than the parent alkane. Justify this statement.
Answer:
Haloalkanes have higher boiling point than the parent alkane having the same number of carbon atoms because the intermolecular forces of attraction and dipole-dipole interactions are comparatively stronger in haloalkanes.
Question 14.
CCl4 > CHCl3 > CH2Cl2 > CH3Cl is the decreasing order of boiling point in haloalkanes. Give reason.
Answer:
The boiling point of chioro, bromo and iodoalkanes increases with increase in the number of halogen atoms. So the correct decreasing order of boiling point of haloalkanes is:
CCl4> CHCl3 > CH2Cl2> CH3Cl.
Question 15.
Arrange the following in increasing order of boiling point. Give reason.
(CH3CH2CH2Cl, CH3CH2Cl, CH3Cl)
Answer:
The correct order of boiling point of haloalkanes is:
CH3CH2CH2Cl > CH3CH2Cl > CH3Cl.
The boiling point of monoalkanes increase with the increase in the number of carbon atoms.
Question 16.
Why haloalkanes arc insoluble in water but soluble in organic solvents?
Answer:
Haloalkanes are polar covalent compounds, soluble in organic solvents, but insoluble in water because they are unable to form hydrogen bond with water molecules.
Question 17.
What happens when bromoethane is treated with moist silver oxide?
Answer:
When bromoethane is treated with moist silver oxide, ethanol will be formed as product:
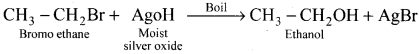
Question 18.
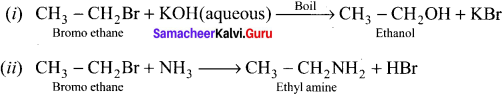
Complete the following reactions:
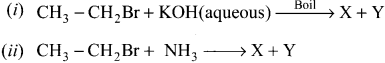
Answer:
Question 19.
Explain the action of sodium hydrogen suiphide with bromoethane?
Answer:
![]()
Question 20.
Explain Williamson’s synthesis.
Answer:
Williamson’s synthesis:
Haloalkanes when boiled with sodium alkoxide gives the corresponding ether.
![]()
Question 21.
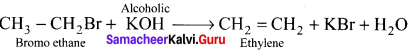
Explain the action of alcoholic potash with bromoethane.
Answer:
Elimination reaction takes place when alcoholic potash reacts with bromoethane:
Question 22.
State-Saytzeff’s rule.
Answer:
Some haloalkanes when treated with alcoholic KOH yield a mixture of olefins with different amounts. It is explained by Saytzeff’s rule which states that in a dehydrohalogenation reaction, the preferreed product is that alkene which has more number ofalkyl group attached to the doubly bonded carbon atom.
Question 23.
How will you convert 1-chioropropanc to propene?
Answer:
![]()
Question 24.
What is Grignard reagent? How is it prepared from ethyl bromide?
Answer:
When a solution of haloalkane in either is treated with magnesium, we will get alkyl magnesium halide known as Grignard reagent, ethyl magnesium bromide is prepared from ethyl bromide as:

Question 25.
How will you prepare ethyl lithium’?
Answer:
When bromoethane is treated with an active metal like lithium in the presence of dry ether, then ethyl lithium will be formed.
![]()
Question 26.
What is tetraethyl Lead? How is it prepared?
Answer:
![]()
Question 27.
Convert bromoethane to ethane.
Answer:
![]()
Question 28.
How is iodoethane converted to ethane?
Answer:
![]()
Question 29.
Starting from CH3MgI, how will you prepare acetaldehyde?
Answer:
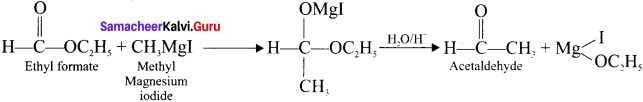
Question 30.
How will you get acetone from methyl manesium iodide?
Answer:
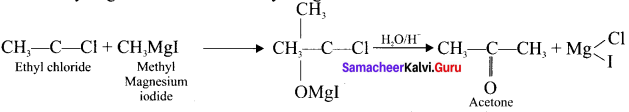
Question 31.
Explain the action of Ethyl chioroformate with Methyl magnesium iodide.
Answer:
Ethyl chioroformate reacts with Grignard reagent to form esters as follows:
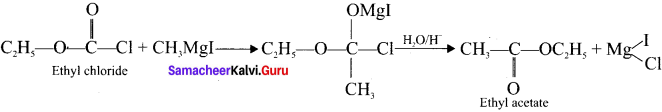
Question 32.
Write the IUPAC names of:

Answer:
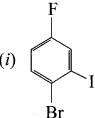 1 – bromo-4-fluoro-2-iodobenzene
1 – bromo-4-fluoro-2-iodobenzene
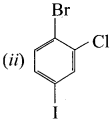 1 – bromo-2-fluoro-4-iodobenzene
1 – bromo-2-fluoro-4-iodobenzene
Question 33.
How is benzene directly converted to chiorobenzene?
Answer:
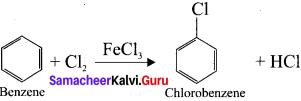
Question 34.
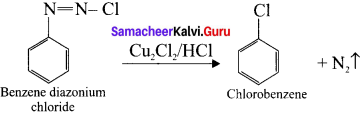
Explain Sandmeyer’s reaction.
Answer:
Sandmeyer’s reaction:
The reaction in which benzene-diazonium chloride is converted to chiorobenzene on heating it with Cu2Cl2/HCl is known as sand Meyer’s reaction.
Question 35.
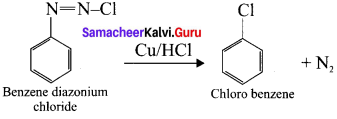
Explain Gattcrrnann reaction.
Answer:
Gattermann reaction:
The reaction in which benzene diazonium chloride react with copper in the presence of HCl, chlorobenzene is formed, called Gatterrnann reaction.
Question 36.
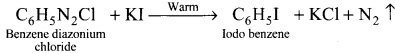
How will you prepare iodobenzene?
Answer:
Question 37.
Explain-Balz-Schiernann reaction.
Answer:
Fluorobenzene is prepared by treating benzene diazonium chloride with fluoro boric acid. This reaction produces diazonium fluoroborate which on heating produce fluorobenzene. this reaction is called Balz-Schiernann reaction.
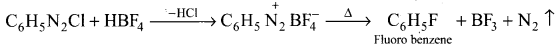
Question 38.
p-dichlorobenzcnc has higher melting point than ortho and meta dichloro benzene. Why?
Answer:
The melting point of p-dichloro benzene is higher than the melting point of the corresponding ortho and mcta isomers. The higher melting which leads to more close packing of its molcules in the crystal lattice and consequently strong intermolecular attractive forces which requires more energy for melting.
p-dichlorobenzene > o-dichlorobenzene > m-dichlorobenzene
Question 39.
Explain Wurtz-fìtting reaction.
Answer:
Wurtz-fitting reaction:
Chlorobenzene and chioroethane are heated with sodium in ether solution to form ethylbenzene. This reaction is called Wurtz-fìtting reaction.
![]()
Question 40.
How will you get benzene from chlorobenzene?
Answer:
Chiorobenzene is reduced with Ni-Al Alloy in the presence of NaOH to give benzene.
![]()
Question 41.
Explain about the preparation of phenyl magnesium chloride.
Answer:
![]()
Question 42.
How will you prepare ethylidcne dichioride from acetylene?
Answer:

Question 43.
What is gern-dihalide? Give one example and explain its preparation.
Answer:
1. If two halogen atoms are attached to one carbon atom of alkyl halide, it is named as gem dihalide. e.g., CH3-CHCl2 (ethylidene dichloride.)
2. Preparationof CH3-CHCl2:

Question 44.
Explain the action of zinc and HCl on chloroform.
Answer:
![]()
Question 45.
How does nickel react with chloroform?
Answer:
![]()
Question 46.
Convert methane to methylene chloride.
Answer:
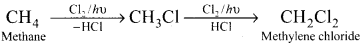
Question 47.
Explain Carbylamine reaction. (or) Give the characteristic test for primary amine.
Answer:
Chlorofonn reacts with aliphatic or aromatic primary amines and alcoholic caustic potash to give foul smelling alkyl isocyanide (carbylamine).
![]()
Question 48.
How will you prepare carbon tetrachioride?
Answer:
The reaction of methane with excess of chlorine in the presence of sunlight give carbon tetrachioride as major product.
![]()
Question 49.
How will you convert carbon tetrachioride to chloroform?
Answer:
![]()
Question 50.
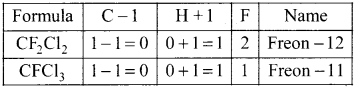
What are freons? How are they named? Give two examples.
Answer:
(i) The chioro-fluoro derivatives of methane and ethane are called freons.
(ii) Freon is represented as freon-cba
c = number of C atoms –
b = number of H atoms + 1
a = total number ofF atoms
Question 51.
What are ambident nucleophiles’? Explain with an example.
Answer:
Nucleophiles which can attack through two different sites are called ambident nucicophiles. For example, cyanide group is a resonance hybrid of two contributing structures and therefore it can act as a nucleophile in two different ways:
![]()
It can attack through carbon to form cyanides and through nitrogen to form isocyanides or carbylamines.
Question 52.
Which compound in each of the following pairs will react faster in SN2 reaction with OH ion?
1. CH3Br or CH3I
2. (CH3)3CCl or CH3Cl
Answer:
1. Since I– ion a better leaving group than Br– ion, therefore CH3I will react faster than CH3Br in SN2 reaction with OH– ion.
2. On steric grounds 1° alkyl halides are more reactive than tert alkyl halide in SN2 reactions. Therefore, CH3Cl will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH– ion.
Question 53.
The treatment ofalkyl chlorides with aqueous KOH solution leads to the formation of alcohols but in the presence of alcoholic KOH solution, alkenes are the major product. Explain.
Answer:
In aqueous solution, KOH is almost completely ioniscd to give OH– ions which being a strong nucleophile brings about a substitution reaction of alkyl halides to form alcohols. In aqueous solution, OH– ions are highly hydrated. This solvation reduces the basic character of OH– ions which therefore, abstract fails to abstract a hydrogen atom from the n-carbon of the alkyl chloride to form an alkene. In contrast, an alcoholic solution of KOH contains alkoxide (RO–) ions which being a much stronger base than OH– ions preferentially eliminates a molecule of HCl from an alkyl chloride to form alkenes.
Question 54.
Give one example of each of the following reactions:
1. Wurtz Reaction
2. Wurtz – Fittig reaction
Answer:
1. Wurtz Reaction:
It involves conversion of alkyl halides into alkane.
Example:
![]()
2. Wurtz-Fittig reaction:
It involves the reaction of an aryl halide and alkyl halide to form the corresponding hydrocarbon.
Question 55.
How will you distinguish between the following pair of compounds:
1. Chloroform and carbon tetrachioride,
2. Benzyl alcohol and chiorobenzene.
Answer:
1. On heating chloroform and carbon tetrachloride with aniline with ethanoic acid and potassium hydroxide separately, chloroform forms a pungent smelling isocyanide compound but carbon tetrachloride does not form this compound.
2. On adding sodium hydroxide and silver nitrate to both the compounds, benzyl chloride forms a white precipitate but chiorobenzene does not form any white precipitate.
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes 3 Marks Questions And Answers
Question 1.
Give one example with structure and name for each of the following compounds.
(a) Primary haloalkane
(b) Secondary haloalkane
(c) Tertiary haloalkane
Answer:
(a) Primary haloalkane
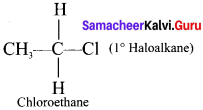
(b) Secondary haloalkane
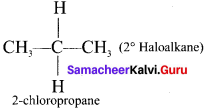
(c) Tertiary haloalkane
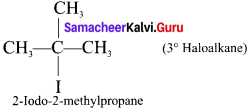
Question 2.
Write the possible isomers for the formula C4H9Cl with structures and names.
Answer:
C4H9Cl (4 isomers):
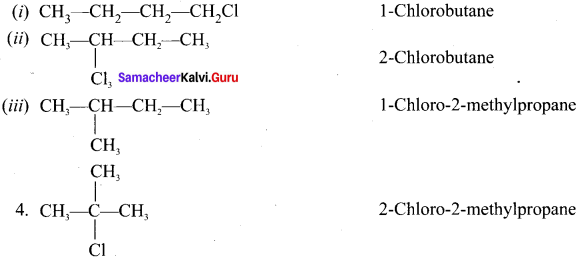
Question 3.
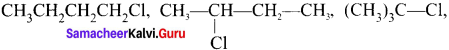
Answer:
For a compound with molecular formula C4H9Cl – 3 isomers (1°, 2°, 3°) arc possible. Among the isomeric alkyl halides, the boiling point decreases with the increase in branching in the alkyl chain. This is because with increase in branching, the molecule attains spherical shape with less surface area. As a result, the intermolecular forces become weak, resulting in lower boiling point. Therefore the boiling point decreases in the order:
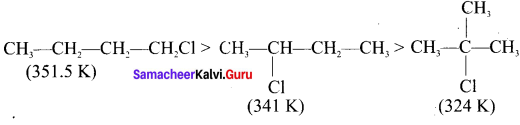
Hence, (CH3)3 C-Cl has the lowest boiling point.
Question 4.
Explain ammonolysis of haloalkanes. (or) How excess of haloalkanc react with alcoholic ammonia?
Answer:
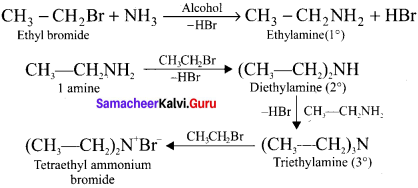
With excess of haloalkanes, ammonia react to give primary, secondary, tertiary amines along with quarternary ammonium salt:
Question 5.
Explain how bromoethane reacts with –
1. alcoholic KCN
2. alcoholic AgCN
Answer:
1. Bromoethanc reacts with alcoholic KCN to form ethyl cyanide.

2. Bromoethane reacts with alcoholic AgCN to form alkyl isocyanide.
![]()
Question 6.
Explain the hydrolysis of 2-bromobutane with aqueous KOB.
Answer:
- 2-bromobutane is optically active and it undergoes SN1 reaction with aqueous KOH.
- The product obtained will be an optically inactive racemic mixture.
- As nucleophilic reagent OH– ion can attack the carbocation from both sides to form equal proportions of dextro and levo rotatory optically active isomers, it results in the formation of optically inactive racemic mixture.
Question 7.
Explain the action of alcoholic KOH with 2-bromobutanc.
Answer:
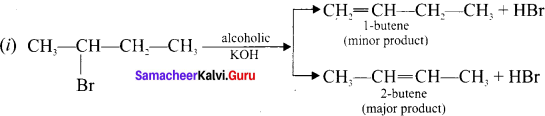
According to Saytzcff’s rule, when 2-bromobutanc reacts with alcoholic KOK, yields a mixture of olefins in different amounts.
(ii) In a dehydrohalogenation reaction, the preferred product is that alkene which has more number of alkyl groups attached to the doubly bonded carbon alkene.
Question 8.
Mention the uses of chloroform.
Answer:
- Chloroform is used as a solvent in pharmaceutical industry.
- It is used for producing pesticides and drugs.
- It is used as an anaesthetic.
Question 9.
What are the uses of carbon tetrachioride?
Answer:
- Carbon tetrachioride in used as a dry cleaning agent.
- It is used as a solvent for oils, fats and waxes.
- As the vapours of CCl4 is non-combustible, it is used under the name pyrene for extinguishing the fire caused by oil (or) petrol.
Question 10.
What are ogranometallic compounds? Give one example. Explain the nature of carbon-metal bond.
Answer:
- Organometallic compounds are organic compounds in which there ¡s a direct carbon-metal bond.
- Example : CH3MgI. methyl magnesium iodide.
- The carbon-magnesium bond in Grignard reagent is covalent but highly polar. The carbon atom is more electronegative than magnesium. Hence, the carbon atom has partial negative charge and magnesium atom has a partial positive charge.

Question 11.
How would you prepare acetic acid from methyl magnesium iodide?
Answer:
Solid carbon dioxide reacts with grignard reagent to form addition product which on hydrolysis yields acetic acid.
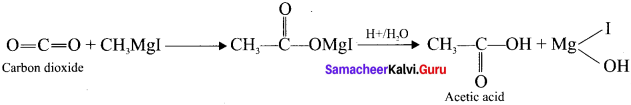
Question 12.
Explain the nature of C-X bond in haloarenes and its resonance structure.
Answer:
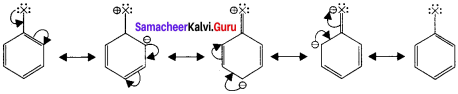
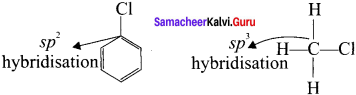
- In haloarenes, the carbon atom is sp2 hybridised. The sp2 hybridised orbitais arc shorter and holds the electron pair bond more.
- Halogen atom contains p-orbital with lone pair of electrons which interacts with π – orbitaIs of berizene ring to form extended conjugated system of π – orbitals.
- The delocalisation of these electrons give double bond character to C-X bond. The resonance structure of halobenzene are given as:
Question 13.
Compare the bond length C-X in haloarenes and C-X in haloalkanes.
Answer:
1. Due to this double bond character of C-X bond in haloarenes, the C-X bond length is shorter length and stronger than in haloalkanes.
2. Example:
Question 14.
What are the uses of chlorobenzene?
Answer:
- Chiorobenzene is used in the manufacture of pesticides like DDT.
- It is used as high boiling solvent in organic synthesis.
- It is used as a fibre – swelling agent in textile processing.
Question 15.
What are polyhalogen compounds? Give its types with example.
Answer:
- Carbon compounds containing more than one halogen atom are called polyhalogen compounds.
- They are classified as
- gem dihalides
- Vicinal dihalides.
(a) Gem dihalide:
In this compound, two halogen atoms are attached to one carbon atom.
e.g, CH3CHCl2 – ethylidene chloride.
(b) Vicinal dihalide:
In this compound, two halogen atoms are attached to two adjacent carbon atoms.
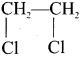 1,2-dichloroethane
1,2-dichloroethane
Question 16.
Give two examples for –
1. gem dihalide
2. vicinal dihalide.
Answer:
1. Gem dihalides:

2. Vicinal dihalides:

Question 17.
Explain two methods of preparation of ethylene diehioride.
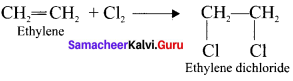
Answer:
1. Addition of Cl2 to Ethylene:
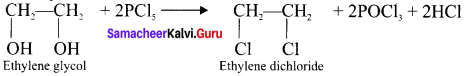
2. Action of PCl5 on Ethylene glycol:
Question 18.
How would you distinguish gern-dihalides and iinal dihalides?
Answer:
(i) Gem-dihalides on hydrolysis with aqueous KOH gives an aldehyde or a ketone whereas vicinal dihalides on hydrolysis with aqueous KOH give glycols.
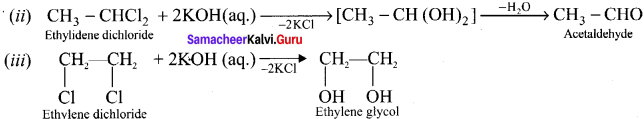
The above reaction can be used to distinguish between gem-dihalides and vicinal dihalides.
Question 19.
Explain the action of metallic zinc with –
(i) Ethylidene dichioride
(ii) Ethylene dichloridc.
Answer:
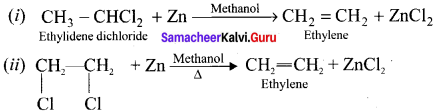
Question 20.
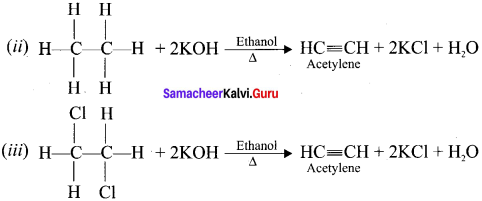
What happens when alcoholic KOH is treated with
(i) Ethylidene dichioride
(ii) Ethylene dich I onde?
Answer:
(i) Gem-dihalides and vicinal dihalides both on treatment with alcoholic KOH give alkynes.
Question 21.
What are the uses of methylene chloride?
Answer:
Methylene chloride is used as:
- Aerosol spray propellant.
- Solvent in paint remover.
- Process solvent in the manufacture of drugs.
- A metal cleaning agent.
Question 22.
Explain the laboratory preparation of chloroform.
Answer:
Haloform reaction:
Chloroform is prepared in the laboratory by the reaction between ethyl alcohol with bleaching powder followed by the distillation of the final product chloroform. Bleaching powder acts as a source of chlorine and calcium hydroxide. The reaction take place in 3 steps.
Step 1:
Oxidation-
![]()
Step 2:
Chlorination-
![]()
Step 3:
Hydrolysis-
![]()
Question 23.
What is chloropicrin? How is it obtained? Mention its uses.
Answer:
1. Chioropicrin is CCl3NO2.
2. Chloroform reacts with nitric acid to form chioropicrin (trichioro-nitromethane):
![]()
3. Chioropicrin is used as an insecticide and soil sterilising agent.
Question 24.
What are the uses of freons?
Answer:
- Freons are used as refrigerants in refrigerators and air conditioners.
- It is used as a propellant for foams and aerosols.
- It is used as a propellant for foams to spray out deodorants, shaving creams and insecticides.
Question 25.
What is DDT? How is it prepared’?
Answer:
(i) DDT is p, p’-dichloro-diphenyi-trichloroethane
(ii) DDT can be prepared by heating a mixture of chloro benzene with chlorai in the presence of conc. H2SO4
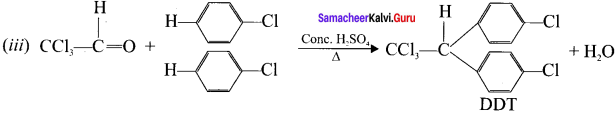
Question 26.
Mention the uses of DDT.
Answer:
- DDT is used lo control certain insects which carries diseases like malaria and yellow fever.
- It is used in farms to control some agricultural pests.
- It is used in building construction as pest control agent.
- It is used to kill certain kind of insects like housefly and mosquitoes due to its high and specific toxicity.
Question 27.
Write the equations for the preparation of I-iodobutane from:
(i) 1 -butanol
(ii) 1 -chiorobutane
(iii) but- 1-ene
Answer:
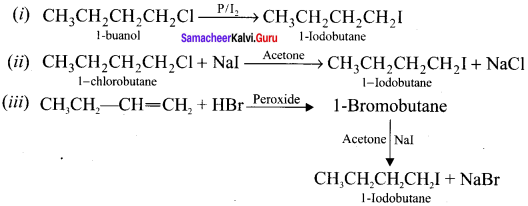
Question 28.
Explain why:
- the dipole moment of chiorobenzene is lower than that of cyclohexyl chloride?
- alkyl halides though polar, are immiscible with water?
- Gringard reagents should be prepared under anhydrous conditions?
Answer:
- Chlorobenzene is stabilised by resonance and there is negative charge on ‘Cl’ in 3 out of 5 resonating structures, therefore it has a lower dipole moment than cyclohexyl chloride in which there is no such negative charge.
- Alkyl halides cannot form H-bond with water and cannot break H-bonds between water molecules, therefore they are insoluble in water.
- Grignard reagents react with H2O to form alkanes, therefore they are prepared under anhydrous conditions.
Question 29.
Explain as to why haloarenes arc much less reactive than haloalkanes towards nucleophilic substitution reactions?
Answer:
Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions due to the following reasons:
1. Resonance effect:
In haloarenes the electron pair on the halogen atom is in conjugation with the π – electrons of the ring and the following resonating structures are possible. C-Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarenes is difficult than in case of haloalkanes and therefore they are less reactive towards nucleophilic substitution reactions.
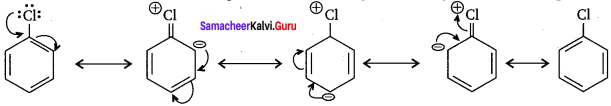
2. The C-Cl bond length in haloalkanes is 177 pm while in haloarenes it is 169 pm. Since it is difficult to break shorter bond than a longer bond. Therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reactions.
Question 30.
Do the following conversions:
(i) Methyl bromide to acetone
(ii) Benzyl alcohol to 2-phenylacetic acid
Answer:
Question 31.
Give reasons for the following:
- Ethyl iodide undergoes SN2 reactions faster than ethyl bromide.
- (±) 2-Butanol is optically inactive.
- C-X bond length in halobenzene is smaller than C-X bond length in CH3-X.
Answer:
- Because in ethyl iodide, iodide being the best leaving group among all the halide ions. Rate of SN2 reaction ability of leaving group.
- (±) 2-hutanol is a racemic mixture which is optically inactive due to the external compensation.
- Due to resonance in halobenzene, it has a smaller bond length value as compared to CH3-X.
Question 32.
Write the structure of diphenyl. How is it prepared from chlorobcnzene?
Answer:
Samacheer Kalvi 11th Chemistry Haloalkanes and Haloarenes 5 Marks Question And Answers
Question 1.
Explain the classification of organic compounds with example.
Answer:
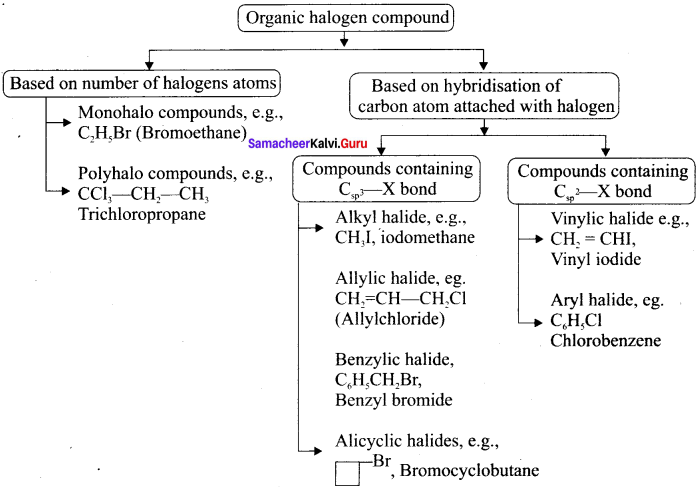
Question 2.
Explain SN2 mechanism with suitable examples.
Answer:
- SN2 reaction means bimolecular nucleophilic substitution reaction of second order.
- The rate of SN2 reaction depends upon the concentration of both alkyl halides and the nucleophile.
- This reaction involves the formation of a transition state in which both the reactant molecules are partially bonded to each other. The attack of nucleophile occurs from the back side.
- The carbon at which substitution occurs has inverted configuration during the course of reaction just as an umbrella has the tendency to invert in a wind-storm. This inversion of configuration is called walden inversion.
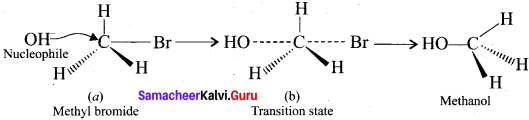
Question 3.
SN2 reaction of an optically active haloalkane is accompanied by inversion of configuration at the asymmetric centre. Prove it.
Answer:
1. SN2 reaction of an optically active haloalkane (e.g., 2-bromo-octane) is accompanied by inversion of configuration at the asymmetric center.
2. When 2-bromooctane is heated with sodium hydroxide, 2-octanol is formed with inversion of configuration. (-) 2-bromo-octane on heating with sodium hydroxide gives (+) 2-octanol is formed in which -OH group occupies a position opposite to what bromine had occupied in the optically active haloalkane.
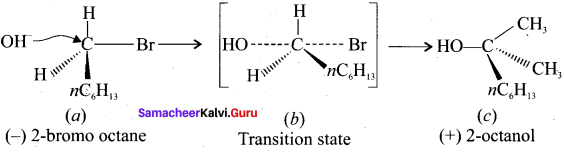
Question 4.
Explain E2 reaction mechanism with a suitable example.
Answer:
- E2 reaction stands bimolecular elimination reaction of second order. The rate of E2 reaction depends on the concentration of alkyl halide and the base.
- Primary alkyl halide undergoes this reaction in the presence of alcoholic KOH.
- It is a one step process in which the abstraction of the proton from the 3 carbon atom and expulsion of halide from the x carbon atom occur simultaneously.
- The mechanism that follows is shown below:

Question 5.
Describe E1 reaction mechanism with a suitable example.
Answer:
1. E1 stands for unimolecular elimination reaction of first order.
2. Tertiary alkyl halides undergoes elimination reaction in the presence of alcoholic KOH.
3. It takes place in two steps.
4. Step 1.
Heterolytic fission to yield a carbocation:
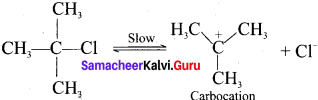
5. Step 2.
Elimination of a proton from the fl-carbon to produce an alkene:
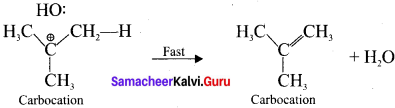
Question 6.
Starting from methyl magnesium iodide how would you prepare –
- Ethanol
- 2-propanol
- Tert-butyl alcohol.
Answer:
1. Ethanol:
Formaldehyde reacts with CH3MgI to give an addition product which on hydrolysis yields ethanol.
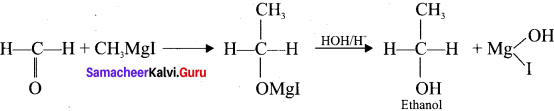
2. 2-Propanol :
Acetaldehyde react with CH3MgI to give an addition product which on hydrolysis yields 2-propanol.
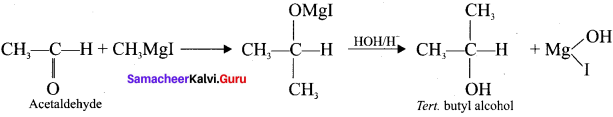
Question 7.
Starting from methyl magnesium iodide, how would you prepare –
- Ethyl methyl ether
- methyl cyanide
- methane.
Answer:
1. Ethyl methyl ether:
Lower halogenated ether reacts with grignard reagent to form higher ether.
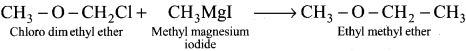
2. Methyl cyanide:
Grignard reagent reacts 4rith cyanogen chloride to form alkyl cyanide.
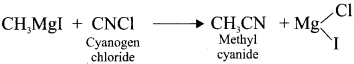
3. Methane:
Grignard reagent reacts with waler to give methane as product.

Question 8.
Describe electrophilic substitution reaction of chiorohenzene with equations.
Answer:
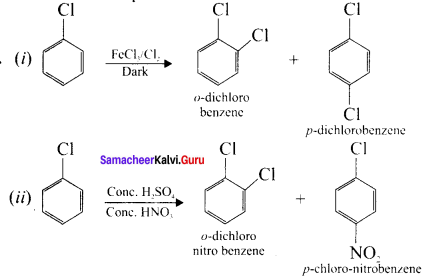
Question 9.
An organic compound Ⓐ of molecular formula C3H6 react with HBr in the presence of peroxide to give C3H7Br as Ⓑ Ⓑ on reaction with aqueous KOH gives © with molecular formula C3H8O. Identify Ⓐ Ⓑ and ©
Answer:
1. An organic compound Ⓐ of molecular formula C3H6 is CH3-CH=CH2, Propene
2. Propene Ⓐ reacts ith HRr in the presence of peroxide to give 1-bromopropane

3. 1-Bromopropane on reaction with aqueous KOH undergoes hydrolysis to give Propan- 1-ol. CH3-CH2-CH2OH as ©
![]()

Question 10.
An organic compound Ⓐ of molecular formula C2H6O reacts with thionyl chloride in the presence of pyridine gives Ⓑ C2H5Cl. Ⓑ on reaction with alcoholic KOH gives Ⓒ, C2H4. Ⓒ on treatmeni with Cl2 gives C2H4Cl2 as Ⓓ. Identify Ⓐ Ⓑ Ⓒ Ⓓ and explain the reaction.
Answer:
1. An organic compound Ⓐ of molecular formula C2H6O is Ethanol CH3-CH2OH.
2. Ethanol reacts with thionyl chloride in the presence of pyridine to give CH3-CH2Cl Ⓑ as product.
![]()
3. Ethyl chloride on treatment with alcoholic KOH, undergoes dehydrohalogenation to give C2H4, ethylene Ⓒ as the product.
![]()
4. Ethylene on reaction with Cl2 yield ethylene dichioride as Ⓓ as the product.
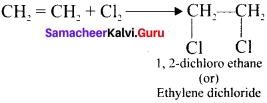
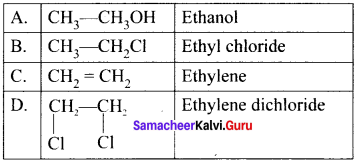
Question 11.
The simplest aromatic hydrocarbon C6H6 reacts Ⓑ on treatment with sodium hydroxide will (C6H5OH), Phenol, Ⓒ as the product. Also Cl2 to give Ⓐ which on reaction with sodium hydroxide gives Ⓑ.Ⓑ of molecular formula C6H6O. Ⓑ on treatment with ammonia will give C6H7N as D. Identify Ⓐ, Ⓑ,Ⓒ, and explain the reactions involved.
Answer:
1. The aromatic hydrocarbon Ⓑ is Benzene, C6H6.
2. Benzene reacts with Cl, to give chiorobenzene (C6H5Cl), as Ⓑ as the product.
![]()
3. Chiorobenzene Ⓑ reacts with sodium hydroxide to give (C6H5OH) phenol, Ⓒ as the product.
![]()
4. Chiorobenzene reacts with ammonia to give Aniline, C6H5NH2 Ⓓ as the product.![]()
Question 12.
An organic compound Ⓐ of molecular formula C2H2 reacts with HCl to give C2H4Cl2 as Ⓑ. Ⓑ on reaction with aqueous KOH will give C2H4O as Ⓒ Identify Ⓐ, Ⓑ,Ⓒ and explain the reactions involved.
Answer:
1. An organic compound Ⓐ of molecular formula C2H2 is CH ≡ CH (Acetylene)
2. Acetylene Ⓐ reacts with HCl to give ethylene dichioride Ⓑ as the product.
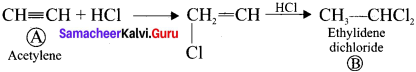
3. Ethylidene dichioride on reaction with aqueous KOH will give acetaldehyde, © as the product.
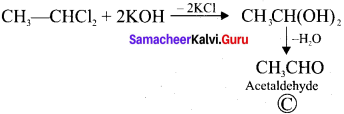
Question 13.
Two isomers of formula C4H9Br are Ⓐ and B. Ⓐ on reaction with alcoholic KOH gives of molecular formula C8H8 by E1 reaction. Ⓑ on reaction with alcoholic KOH gives Ⓓ and Ⓔ as products by Saytzefì’s rule. Idcnti’ A, B, C, D, E.
Answer:
1. C4H9Br: Two isomers may be there: 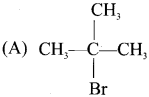 Tert butyl bromide and
Tert butyl bromide and 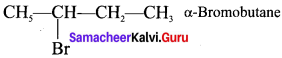
2. Ⓐ on reaction with alcoholic KOH gives iso butylene © as the product.
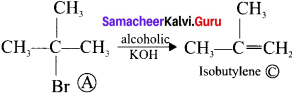
3. 2-bromobutane Ⓑ on reaction with alcoholic KOH follows Saytzeff s rule to give a mixture of olefins in different amounts.
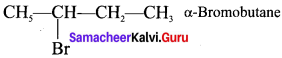

Question 14.
A simple aromatic hydrocarbon Ⓐ reacts with Cl2 to give Ⓑ of molecular formula C6H5Cl. Ⓑ on reaction with ethyl chloride along with sodium metal gives © of formula C8H10. © alone reacts with Na metal in the presence of ether to give Ⓓ C12H10. Identify Ⓐ Ⓑ Ⓒ & Ⓓ
Answer:
1. The simple aromatic hydrocarbon is C6H6. Benzene (A)
2. Benzene reacts with Cl2 to give chiorobenzene.
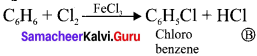
3. Chiorobenzene reacts with ethyl chloride to form ethyl benzene, © as the product
![]()
4. Chiorobenzene on reaction Na metal gives Biphenyl compound © as the product.
![]()
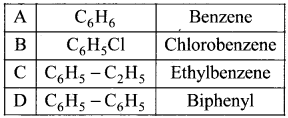
Question 15.
An organic compound Ⓐ of molecular formula CH2O reacts with methyl magnesium iodide followed by acid hydrolysis to give Ⓑ of moLecular formula C2H6O. Ⓑ on reaction with PCl5 gives Ⓑ. © on reaction with alcoholic KOK gives Ⓓ an alkene as the product. Identif’ Ⓐ Ⓑ Ⓒ Ⓓ and explain the reactions involved.
Answer:
1. Ⓐ of molecular formula CH2O is identified as HCHO, formaldehyde.
2. Formaldehyde reacts with CH3MgI followed by hydrolysis to give ethanol, CH3-CH2OH B as the product.
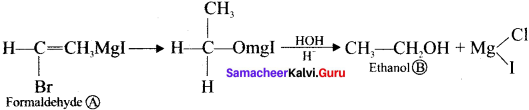
3. Ethanol Ⓑ reacts with PCl5 to give C2H5Cl, Ethyl chloride © as the product.
![]()
4. CH3-CH2Cl © on reaction with alcoholic KOH undergoes dehydrohalogenation to give ethylene CH2=CH3 Ⓓ as the product.
![]()
We believe the provided Tamilnadu State Board for Class 11th Chemistry Solutions Chapter 14 Haloalkanes and Haloarenes Guide Pdf Free Download will benefit you. In case, do you have any questions regarding Samacheer Kalvi 11th Chemistry Solutions Book Solutions Chapter 14 Haloalkanes and Haloarenes Pdf, Questions and Answers, leave a comment below and we will get back to you at the earliest. Meanwhile, Bookmark our site for more information on State Board Solutions for various subjects.