Tamilnadu State Board Solutions for Class 12th Chemistry Solutions Chapter 7 Chemical Kinetics Questions and Answers will help you to improve the complete subject knowledge. Every student must look at the single concept included in Samacheer Kalvi 12th Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions Answers Guide Solutions PDF. All the concepts are explained clearly with examples and pictures.
Students can easily avoid the struggle to get the best book for Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions Answers Guide learning. You have to go through Chapterwise Samacheer Kalvi Class 12th Textbook Solutions for Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions for better practice.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 7 Chemical Kinetics
Students who wish to have the strong basics of Chemistry Solutions Chapter 7 Chemical Kinetics can use Tamilnadu State Board Class 12th Chemistry Solutions Chapter 7 Chemical Kinetics Questions and Answers Guide pdf. Enhance your knowledge by referring to the Samacheer Kalvi Solutions pdf. We provided a free pdf of Tamilnadu State Board Class 12th Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions Answers Guide material and textbook for students. Check it out now and start preparing for the exam immediately. Score maximum marks in the exam by referring to Samacheer Kalvi Class 12th Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions Answers Guide Solutions Pdf.
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements Text Book Evalution
I. Choose the correct answer.
12th Chemistry Chapter 7 Book Back Answers Question 1.
For a first order reaction A → B the rate constant is x min-1. If the initial concentration of A is 0.01 M, the concentration of A after one hour is given by the expression.
(a) 0.01 e-x
(b) 1 x 10-2 (1 – e-60x)
(c) (1 x 10-2) e-60x
(d) none of these
Answer:
(c) (1 x 10-2) e-60x
Answer:
Solutions:
In this case
k = x min-1 and [A0] = 0.01 M
= 1 x 10-2 M
t = 1 hour = 60 min
[A] = 1 x 10-2(e-60x)
12th Chemistry 7th Lesson Book Back Answers Question 2.
A zero order reaction X → Product, with an initial concentration 0.02M has a half life of 10 min. If one starts with concentration 0.04M, then the half life is …………….
(a) 10 s
(b) 5 min
(c) 20 min
(d) cannot be predicted using the given information
Answer:
(c) 20 min
Solutions:
Given,
[A0] = 0.02 M ; t1/2 = 10 min
[A0] = 0.04 M ; t1/2 = ?
Substitute in (1)
10 min ? 0.02 M ……………………..(2)
t1/2 ∝ 0.04 M ……………………..(3)
Dividing Eq.(3) by Eq. (2) we get,
\(\frac { { t }^{ 1/2 } }{ 10min }\) = \(\frac { 0.04M }{ 0.02M }\)
t1/2 = 2 x 10 min = 20 min
Samacheer Kalvi Guru 12th Chemistry Question 3.
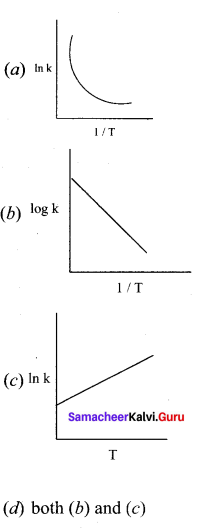
Among the following graphs showing variation of rate constant with temperature (T) for a reaction, the one that exhibits Arrhenius behavior over the entire temperature range is ……………
Answer:
Solution:
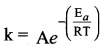
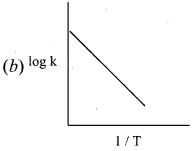
In k = In A – \(\left( \frac { { E }_{ a } }{ R } \right)\) \((\frac { 1 }{ T })\)
this equation is in the form of a straight
line equation y = c + m x
a plot of ink vs \((\frac { 1 }{ T })\) is a straight line with negative slope.
12th Chemistry Lesson 7 Book Back Answers Question 4.
For a first order react ion A → product with initial concentration x mol L-1, has a half life period of 2.5 hours. For the same reaction with initial concentration mol L-1 the half life is
(a) (2.5 x 2) hours
(b) \((\frac { 2.5 }{ 2 })\) hours
(c) 2.5 hours
(d) Without knowing the rate constant, t1/2 cannot be determined from the given data
Answer:
(d) Without knowing the rate constant, t1/2 cannot be determined from the given data.
Solutions:
For a first order reaction
t1/2 = \(\frac { 0.693 }{ k }\) t1/2 does not depend on the initial concentration and it remains constant (whatever may be the initial concentration)
t1/2 = 2.5 hrs .
Question 5.
For the reaction, 2NH3 → N2 + 3H2, if
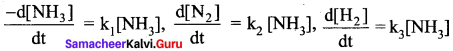
then the relation between
k1, k2 and k3 is
(a) k1 = k2 = k3
(b) k1 = 3 k2 = 2 k3
(c) 1.5k1 = 3 k2 = k3
(d) 2k1 = k2 = 3 k3
Answer:
(c) 1.5k1 = 3 k2 = k3
Solution:
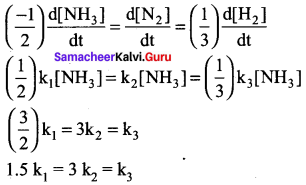
12th Chemistry Book Inside Evaluate Yourself Answers Question 6.
The decomposition of phosphine (PH3) on tungsten at low pressure is a first order reaction. It is because the …………….
(a) rate is proportional to the surface coverage
(b) rate is inversely proportional to the surface coverage
(c) rate is independent of the surface coverage
(d) rate of decomposition is slow
Answer:
(c) rate is independent of the surface coverage
Solution:
Given:
At low pressure the reaction follows first order, therefore Rate ∝ [reactant]1 Rate ∝ (surface area) At high pressure due to the complete coverage of surface area, the reaction follows zero order. Rate ∝ [reactant]°. Therefore the rate is independent of surface area.
Samacheer 12 Chemistry Solutions Question 7.
For a reaction Rate = k [acetone]3/2 then unit of rate constant and rate of reaction respectively is …………..
(a) (mol L-1 s-1), (mol-1/2 L1/2 s-1)
(b) (mol-1/2 L1/2 s-1), (mol L-1 s-1)
(c) (mol1/2 L1/2 s-1), (mol L-1 s-1)
(d) (mol L s-1), (mol1/2 L1/2 s)
Answer:
(b) (mol1/2 L1/2 s-1), (mol L-1 s-1)
Solution:
Rate = k [A]n
Rate = \(\frac { -d[A] }{ dt } \)
unit of rate = \(\frac { mol{ L }^{ -1 } }{ s }\) = mol L-1 s-1
unit of rate constant = \(\frac { (mol{ L }^{ -1 }{ S }^{ -1 }) }{ { (mol{ L }^{ -1 }) }^{ n } }\)
= mol1-n Ln-1 S-1
in this case, rate k [Acetone]3/2
n = 3/2
mol1-(3/2) L(3/2)-1 s-1
mol-(1/2) L(1/2) s-1
12th Chemistry Evaluate Yourself Answers Samacheer Question 8.
The addition of a catalyst during a chemical reaction alters which of the following quantities?
(a) Enthalpy
(b) Activation energy
(c) Entropy
(d) Internal energy
Answer:
(b) Activation energy
Solution:
A catalyst provides a new path to the reaction with low activation energy. i.e., it lowers the activation energy.
12 Chemistry Evaluate Yourself Answers Question 9.
Consider the following statements:
(i) increase in concentration of the reactant increases the rate of a zero order reaction.
(ii) rate constant k is equal to collision frequency A if Ea = o
(iii) rate constant k is equal to collision frequency A if Ea = o
(iv) a plot of ln (k) vs T is a straight line.
(v) a plot of In (k) vs \((\frac { 1 }{ T })\) is a straight line with a positive slope.
Correct statements are
(a) (ii) only
(b) (ii) and (iv)
(c) (ii) and (v)
(d) (i), (ii) and (v)
Answer:
(a) (ii) only
Solutions:
In zero order reactions, increase in the concentration of reactant does not alter the rate, So statement (i) is wrong.

if Ea = O (so, statement (ii) is correct, and statement (iii) is wrong)
k = A e°
k = A
in k = A – \(\left( \frac { { E }_{ a } }{ R } \right)\) \(\frac { 1 }{ T }\)
this equation is in the form of a straight line equation yc + m x. a plot of Ink vs \(\frac { 1 }{ T }\) is a straight line with negative slope so statements (iv) and (v) are wrong.
Evaluate Yourself 12th Chemistry Question 10.
In a reversible reaction, the enthalpy change and the activation energy in the forward direction are respectively – x kJ mol-1 and y kJ mol-1. Therefore, the energy of activation in the backward direction is ………..
(a) (v – x)kJ mol-1
(b) (x + y) J mol-1
(c) (x – y) kJ mol-1
(d) (x + y) x 103 J mol-1
Answer:
(d) (x + y) x 103 J mol-1
Solution:
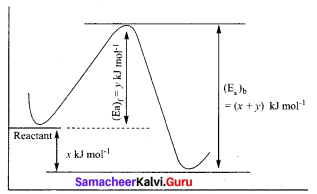
Chemistry Class 12 Samacheer Kalvi Question 11.
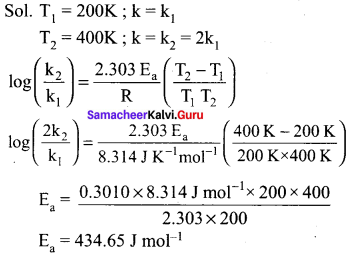
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 200K to 400K? (R 8.314 JK-1 mol-1)
(a) 234.65 kJ mol-1 K-1
(b) 434.65 kJ mol-1 K-1
(c) 434.65 J mol-1 K-1
(d) 334.65 J mol-1 K-1
Answer:
(c)434.65 J mol-1 K-1
Solutions:
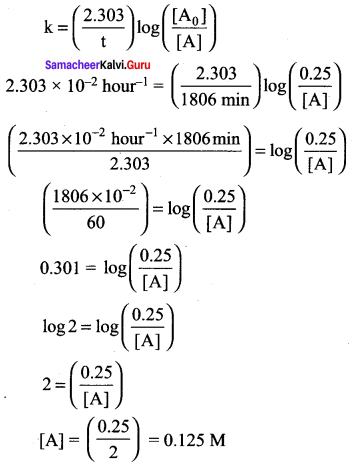
12th Chemistry Solution Book Question 12.
![]()
This reaction follows first order kinetics. The rate constant at particular temperature is 2.303 x 102 hourd. The initial concentration of cyclopropane is 0.25 M. What will be the concentration of cyclopropane after 1806 minutes? (Log 2 = 0.30 10)
(a) 0.125 M
(b) 0.215 M
(c) 0.25 x 2.303 M
(d) 0.05 M
Answer:
(b) 0.2 15 M
Solution:
12th Chemistry Evaluate Yourself Answers Question 13.
For a first order reaction, the rate constant is 6.909 min-1.The time taken for 75% conversion in minutes is …………
(a) \((\frac { 3 }{ 2 })\) log 2
(b) \((\frac { 3 }{ 2 })\) log 2
(c) \((\frac { 3 }{ 2 })\) log \((\frac { 3 }{ 4 })\)
(d) \((\frac { 2 }{ 3 })\) log \((\frac { 4 }{ 3 })\)
Answer:
(b) \((\frac { 3 }{ 2 })\) log 2
Solution:
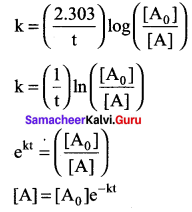
k = \((\frac { 2.303 }{ t })\) log \(\left( \frac { \left[ { A }_{ 0 } \right] }{ \left[ A \right] } \right)\)
[A0] = 100
[A] = 25
[A0]= 100; [A]=25
6.909 = \((\frac { 2.303 }{ t })\) log \((\frac { 100 }{ 25 })\)
t = \((\frac { 2.303 }{ 6.909 })\) log (4) ⇒ t = \((\frac { 1 }{ 3 })\) log 22
t = \((\frac { 2 }{ 3 })\) log 2
Samacheerkalvi.Guru 12th Chemistry Question 14.
In a first order reaction x → y; if k is the rate constant and the initial concentration of the reactant x is 0.1 M, then, the half life is ……..
(a) \((\frac { log2 }{ k })\)
(b) \((\frac { 0.693 }{ (0.1)k })\)
(c) \((\frac { In2 }{ k })\)
(d) none of these
Answer:
(c) \((\frac { In2 }{ k })\)
Solution:
k = \((\frac { 1 }{ t })\) In \(\left( \frac { \left[ { A }_{ 0 } \right] }{ \left[ A \right] } \right)\)
[A0] = 0.1
[A] = 0.05
k = \(\left( \frac { 1 }{ { t }_{ 1/2 } } \right)\) In \((\frac { 0.1 }{ 0.05 })\)
k = \(\left( \frac { 1 }{ { t }_{ 1/2 } } \right)\) In (2) ⇒ t1/2 = \((\frac { In(2) }{ k })\)
12 Chemistry Solutions Samacheer Question 15.
Predict the rate law of the following reaction based on the data given below:
2A + B → C + 3D
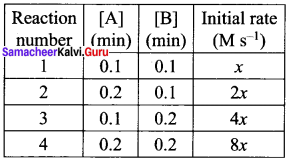
(a) rate = k [A]2 [B]
(b) rate = k [A][B]2
(c) rate = k [A][B]
(d) rate = k [A]1/2 [B]3/2
Answer:
(b) rate = k [A][B]2
Solution:
rate1 = k [0.1]n [0.1]m ……………(1)
rate2 = k [0.2]n [0.1]m …………(2)
Dividing Eq.(2) by Eq.(1)
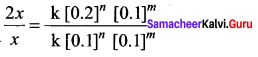
\(\frac { 2x }{ x }\) = 2n
∴ n = 1
rate3 = k [0.1]n [0.2]m …………..(3)
rate4 = k [0.2]n [0.2]m …………..(4)
Dividing Eq.(4) by Eq.(2)
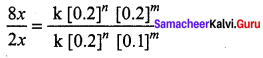
\(\frac { 8 }{ 2 } \) = 2m
∴m = 1
∴ rate = k [A]1 [B]2
12th Chemistry Solutions Samacheer Question 16.
Assertion: rate of reaction doubles when the concentration of the reactant is doubles if it is a first order reaction.
Reason: rate constant also doubles
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Solution:
For a first reaction, when the concentration of reactant is doubled, then the rate of reaction also doubled. Rate constant is independent of concentration and is a constant at a constant temperature, i.e., it depends on the temperature and hence, it will not be doubled and when the concentration of the reactant is doubled.
Chapter 7 Chemistry Class 12 Question 17.
The rate constant of a reaction is 5.8 x 102 s1. The order of the reaction is ………….
(a) First order
(b) zero order
(c) Second order
(a) Third order
Answer:
(a) First order
Solution:
The unit of rate constant is s-1 and it indicates that the reaction is first order.
Samacheer Kalvi Class 12 Chemistry Solutions Question 18.
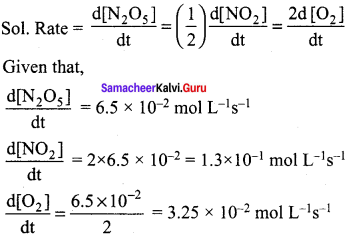
For the reaction N2 O5(g) → 2NO2(g) +\(\frac { 1 }{ 2 }\) – O2(g) the value of rate of disappearance of N2O5 is given as 6.5 x 10-2 mol L-1s-1 The rate of formation of NO2 and O2 is given respectively as …………….
(a) (3.25 x 10-2 mol L-1s-1) and (1.3 x 10-2 mol L-1s-1)
(b) (1.3 x 10-2 mol L-1s-1) and (3.25 x 102 mol L-1s-1)
(c) (1.3 x 10-1 mol L-1s-1) and (3.25 x 10-2 mol L-1s-1)
(d) None of these
Answer:
(c) (1.3 x 10-1 mol L-1s-1) and (3.25 x 10-2 mol L-1s-1)
Question 19.
During the decomposition of H2O2 to give dioxygen, 48g O2 is formed per minute at certain point of time. The rate of formation of water at this point is …………….
(a) 0.75 mol min-1
(b) 1.5 mol min-1
(c) 2.25 mol min-1
(d) 3.0 mol min-1
Answer:
(d) 3.0 mol min-1
Solution:
H2O2 → H2O + \(\frac { 1 }{ 2 }\)O2
Rate = ![]()
No. of moles of oxygen = \((\frac { 48 }{ 32 })\) = 1.5 mol
Rate of formation of oxygen = 2 x 1.5
= 3 mol min-1
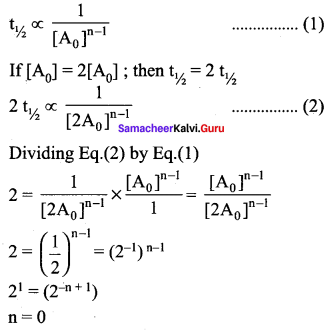
Question 20.
If the initial concentration of the reactant is doubled, the time for half reaction is also doubled. Then the order of the reaction is …………
(a) Zero
(b) one
(c) Fraction
(d) none
Answer:
(a) Zero
Solution:
For a first order reaction t1/2 is independent of initial concentration .i.e., n \(\neq\) 1 for such cases
Question 21.
In a homogeneous reaction A ? B + C + D, the initial pressure was P0 and after time t it was P. Expression for rate constant in terms of P0, P and t will be ……….
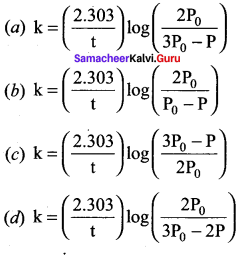
Answer:
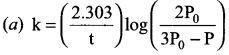
Solution:

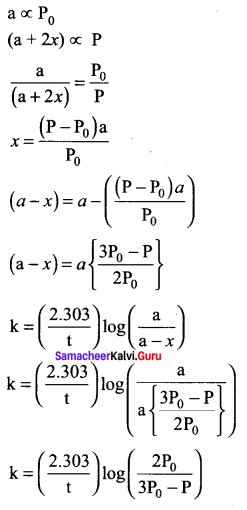
Question 22.
If 75% of a first order reaction was completed in 60 minutes, 50% of the same reaction under the same conditions would be completed in ………
(a) 20 minutes
(b) 30 minutes
(c) 35 minutes
(d) 75 minutes
Answer:
(b) 30 minutes
Solution:

Question 23.
The half life period of a radioactive element is 140 days. After 560 days, 1 g of element will be reduced to
(a) \(\frac { 1 }{ 2 }\) g
(b) \(\frac { 1 }{ 4 }\) g
(c) \(\frac { 1 }{ 8 }\) g
(d) \(\frac { 1 }{ 16 }\) g
Answer:
(d) \(\frac { 1 }{ 16 }\) g
Solution:
in 140 days ⇒ initial concentration reduced to \(\frac { 1 }{ 2 }\) g
in 280 days ⇒ initial concentration reduced to \(\frac { 1 }{ 4 }\) g
in 420 days ⇒ initial concentration reduced to \(\frac { 1 }{ 8 }\) g
in 560 days ⇒ initial concentration reduced to \(\frac { 1 }{ 8 }\) g
Question 24.
The correct difference between first and second order reactions is that …………
(a) A first order reaction can be catalysed a second order reaction cannot be catalysed.
(b) The half life of a first order reaction does not depend on [A0] the half life of a second order reaction does depend on [A0].
(c) The rate of a first order reaction does not depend on reactant concentrations; the rate of a second order reaction does depend on reactant concentrations.
(d) The rate of a first order reaction does depend on reactant concentrations; the rate of a second order reaction does not depend on reactant concentrations,
Answer:
(b) The half life of a first order reaction does not depend on [A0]; the half life of a second order reaction does depend on [A0].
Solution:
For a first order reaction
t1/2 = \(\frac { 0.6932 }{ k }\)
For a second order reaction
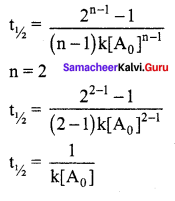
Question 25.
After 2 hours, a radioactive substance becomes \((\frac { 1 }{ 16 })\)th of original amount. Then the half life (in mm) is ………………
(a) 60 minutes
(b) 120 minutes
(c) 30 minutes
(d) 15 minutes
Answer:
(c) 30 minutes
Solution:

II . Answer the following questions:
Question 1.
Define average rate and instantaneous rate.
Answer:
1. Average rate:
The average rate of a reaction is defined as the rate of change of concentration of a reactant (or of a product) over a specified measurable period of time.
2. insantaneous rate:
Instantaneous rate of reaction gives the tendency of the reaction at a particular point of time during its course (or) The time derivative of the concentration of a reactant (or product) converted to a positive number is called the instantaneous rate of reaction.
Question 2.
Define rate law and rate constant.
1. Rate law:
The expression in which reaction rate is given in terms of molar concentration of the reactants with each term raised to some power, which may or may not be same as the Stoichiometric coefficient of the reacting species in a balanced chemical equation.
x A + y B → products
Rate = k [A]m [B]m
k = Rate constant
2. Rate constant:
For a reaction involving the reactants A and B, Reaction rate = k [A]m [B]m The constant k is called rate constant of the reaction. If [A] = 1 M and [B] = 1 M; Reaction rate = k Thus, the rate constant (k) of a reaction is equal to the rate of reaction when the concentration of each reactant is equal to 1 mol L-1. The change in the concentration of reactant or product per unit time under the condition of unit concentration of all the reactant.
Question 3.
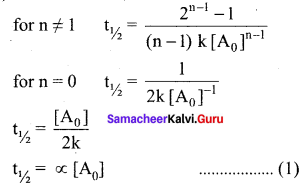
Derive integrated rate law for a zero order reaction A product. A reaction in which the rate is independent of the concentration of the reactant over a wide range of concentrations is called as zero order reactions. Such reactions are rare. Let us consider the following hypothetical zero order reaction.
A → Product
The rate law can be written
Rate = k [A]°
(∴[A]° = 1)
– d [A] k (l)
\(\frac { -d[A] }{ dt }\) = k(1)
-d[A] = k dt
Integrate the above equation between the limits of [A0] at zero time and [A] at some later time ‘t’,

Question 4.
Define half life of a reaction. Show that for a first order reaction half life is independent of Initial concentration.
Answer:
Half life of a reaction is defined as the time required for the reactant concentration to reach one half of its initial value. For a first order reaction, the half life is a constant i.e., it does not depend on the initial concentration. The rate constant for a first order reaction is given by,
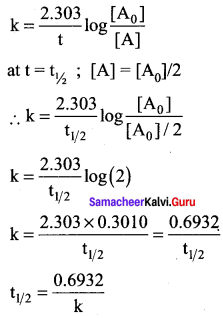
Question 5.
What is an elementary reaction? Give the differences between order and molecularity of a reaction.
Answer:
Elementary reaction – Each and every single step in a reaction mechanism is called an elementary reaction. Differences between order and molecularity:
Order of a reaction:
- It is the sum of the powers of concentration terms involved in the experimentally determined rate law.
- It can be zero (or) fractional (or) integer.
- It is assigned for a overall reaction.
Molecularity of a reaction:
- It is the total number of reactant species that are involved in an elementary step.
- It is always a whole number, cannot be zero or a fractional number.
- It is assigned for each elementary step of mechanism.
Question 6.
Explain the rate determining step with an example.
Answer:
1. Most of the chemical reactions occur by multistep reactions. In the sequence of steps it is found that one of the steps is considerably slower than the others. The overall rate of the reaction cannot be lower in value than the rate of the slowest step.
2. Thus in a multistep reaction the experimentally determined rate corresponds to the rate of the slowest step. The step which has the lowest rate value among the other steps of the reaction is called as the rate determining step (or) rate limiting step.
3. Consider the reaction,
2A + B → C + D
going by two steps as follows,

Here the overall rate of the reaction corresponds to the rate of the first step which is the slow step and thus the first step is called as the rate determining step of the reaction. In the above equation, the rate of the reaction depends upon the rate constant k ( only. The rate of second step dosn’t contribute experimentally determined overall rate of the reaction.
For example,
NO2(g) + CO2(g) → NO(g) + CO2(g)
Which occurs in two elementary steps:
- NO2 + NO2 → NO + NO3 (Slow)
- NO3+ CO → NO2 + CO2 (fast)
Because the first step is the lowest step, the overall reaction cannot proceed any faster than the rate of the first elementary step. The first elementary step in this example is therefore the rate determining step.
The rate equation for this reaction is equal to the rate is constant of step-1 multiplied by the reactants of that first step. If the rate constant of step-1 is denoted as k1 then the rate of the first step in the reaction (and the total reaction) will be,
Rate = k, [NO2] [NO2]
= k1 [NO2]2
Question 7.
Describe the graphical representation of first order reaction.
Answer:
Rate constant for first order reaction is,
kt = ln\(\left( \frac { \left[ { A }_{ 0 } \right] }{ \left[ A \right] } \right)\)
kt = In [A0] – In [A]
In[A] = In [A0] – kty = c + mx
If we follow the reaction by measuring the concentration of the reactants at regular time interval ‘t’, a plot of ln[A] against ‘t’ yields a straight line with a negative slope. From this, the rate constant is calculated.
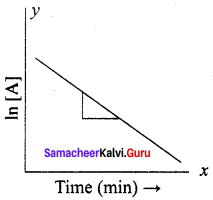
Question 8.
Write the rate law for the following reactions.
- A reaction that is 3/2 order in x and zero order in y.
- A reaction that is second order in NO and first order in Br2.
Answer:
1. \(\frac { 3 }{ 2 }\) x + y (excess) → products
– \(\frac { 3 }{ 2 }\) \(\frac { d[x] }{ dt }\) = k [x]3/2
2. 2NO + Br2 → products
– \(\frac { 1 }{ 2 }\) \(\frac { d[NO] }{ dt }\) = k [NO]2 [Br2]
Question 9.
Explain the effect of catalyst on reaction rate with an example.
Answer:
- Significant changes in the reaction can be brought out by the addition of a substance called catalyst.
- A catalyst is substance which alters the rate of a reaction without itself undergoing any permanent chemical change.
- They may participate in the reaction, but again regenerated and the end of the reaction.
- In the presence of a catalyst, the energy of activation is lowered and hence, greater number of molecules can cross the energy barrier and change over to products, thereby increasing the rate of the reaction.
- For example, decomposition of potassium chlorate is enhanced by addition of MnO2.
2KClO3 \(\frac{\mathrm{MnO}_{4}}{\Delta} 2 \mathrm{KCl}\) + 3O2 (MnO2 – Catalyst)
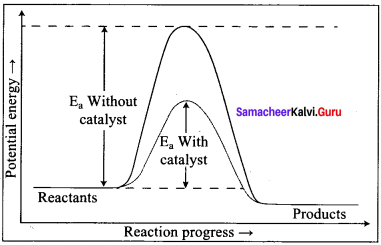
Question 10.
The rate law for a reaction of A, B and C has been found to be rate = k[A]2 [B][L]3/2. How would the rate of reaction change when
- Concentration of [L] is quadrupled
- Concentration of both [A] and [B] are doubled
- Concentration of [A] is halved
- Concentration of [A] is reduced to(1/3) and concentration of [L] is quadrupled.
Solution:
Rate = k [A]2 [B] [L]3/2 ………….(1)
1. when [L] = [4L]
Rate = k [A]2 [B] [4L]3/2
Rate = 8 (k[A]2 [B] [L]3/2) …………………..(2)
Comparing (1) and (3) rate is increased by 8 times.
2. when [A] = [2A] and [B] = [2B]
Rate = k[2A]2 [2B ] [L]3/2
Rate = 8 (k[A]2 [B] [L]3/2 …………….(3)
Comparing (1) and (3); rate is increased by 8 times.
3. when [A] = \([\frac { A }{ 2 }]\)
Rate = k \([\frac { A }{ 2 }]\)2 [L]\(\frac { 3 }{ 2 }\)
Rate = \(\frac { 1 }{ 4 }\) (k[A]2 [B] [L]3/2) ……………..(4)
Comparing (1) and ( 4); rate is reduced to \(\frac { 1 }{ 4 }\) times.
4. when [A] = \([\frac { A }{ 3 }]\) and [L] = [4L]
Rate k\(\frac { A }{ 3 }\)2 [B] [4L]3/2
Rate = \([\frac { 8 }{ 9 }]\) (k[A]2 [B] [L]3/2) ……………….(5)
Comparing (1) and (5); rate is reduced to 8/9 times.
Question 11.
The rate of formation of a dimer in a second order reaction is 7.5 x 10-3 mol L-1s-1 at 0.05 mol L-1 monomer concentration. Calculate the rate constant.
Solution:
Let us consider the dimensation of a monomer M
2M → (M)2
Rate = k [M]n
Given that n =2 and [M] = 0.05 mol L-1
Rate = 7.5 x 10-3 mol L-1s-1
Rate 7.5 x 103 mol L-1 s-1
k = \(\frac { Rate }{ { \left[ M \right] }^{ n } }\)
k= =\(\frac { 7.5\times { 10 }^{ -3 } }{ { \left( 0.05 \right) }^{ 2 } }\) = 3 mol-1 Ls-1
Question 12.
For a reaction x +y + z → products, the rate law is given by rate = k [x]3/2 [y]1/2 what is the overall order of the reaction and what is the order of the reaction with respect to z.
Solution:
Rate = k [x]3/2 [y]1/2
overall order = \(\left( \frac { 3 }{ 2 } +\frac { 1 }{ 2 } \right)\) = 2
i.e., second order reaction.
Since the rate expression does not contain the concentration of Z , the reaction is zero order with respect to Z.
Question 13.
Explain briefly the collision theory of bimolecular reactions.
Answer:
Collision theory is based on the kinetic theory of gases. According to this theory, chemical reactions occur as a result of collisions between the reacting molecules. Let us understand this theory by considering the following reaction.
A2(g) + B2(g) → 2AB(g)
If we consider that, the reaction between A2 and B2 molecules proceeds through collisions between them, then the rate would be proportional to the number of collisions per second. Rate ix Number of molecules colliding per litre per second (or) Rate ∝ Collision rate. The number of collisions is directly proportional to the concentration of both A2 and B2.
Collision rate ∝ [A2] [B2] …………………(1)
Collision rate = Z [A2] [B2] ……………………..(2)
Where, Z is a constant.
The collision rate ¡n gases can be calculated from kinetic theory of gases. For a gas at room temperature (298K) and 1 atm pressure, each molecule undergoes approximately 109 collisions per second, i.e., I collision in 109 second. Thus, if every collision resulted in reaction, the reaction would be complete in 109 second.
In actual practice this does not happen. It implies that all collisions are not effective to lead to the reaction. In order to react, the colliding molecules must possess a minimum energy called activation energy. The molecules that collide with less energy than activation energy will remain intact and no reaction occurs.
Fraction of effective collisions (f) is given by the following expression, \({ e }^{ \frac { { -E }_{ a } }{ RT } }\)
Fraction of collisions is further reduced due to orientation factor i.e., even if the reactant collide with sufficient energy, they will not react unless the orientation of the reactant molecules is suitable for the formation of the transition state. The fraction of effective collisions (f) having proper orientation is given by the steric factor P.
Rate = P x f x collision rate
Rate= P x \({ e }^{ \frac { { -E }_{ a } }{ RT } }\) x Z [A2] [B2] ……..(1)
As per the rate law, Rate = k [A2] [B2] ………………….(2)
Where k is the rate constant
On comparing equation (1) and (2), the rate constant k is,
k = p Z \({ e }^{ \frac { { -E }_{ a } }{ RT } }\)
Question 14.
Write Arrhenius equation and explains the terms involved.
Answer:
Arrhenius equation:
k = A\({ e }^{ \frac { { -E }_{ a } }{ RT } }\)
A = Arrhenius factor (frequency factor)
R = Gas constant
k = Rate constant
Ea = Activation energy
T = Absolute temperature (in K)
Question.15.
The decomposition of Cl2O7 at 500K in the gas phase to Cl2 and O2 is a first order reaction. After 1 minute at 500K, the pressure of Cl2O7 falls from 0.08 to 0.04 atm. Calculate the rate constant in s-1.
Answer:
Solution:
k = \(\frac { 2.303 }{ t }\) log \(\frac{\left[\mathrm{A}_{0}\right]}{[\mathrm{A}]}\)
k = \(\frac { 2.303 }{ 1 min }\) log \(\frac { [0.08] }{ [0.04] }\)
k = 2.303 log 2
k = 2.303 x 0.3010
k = 0.693 2 min-1
k = \((\frac { 0.6932 }{ 60 })\) s-1
k = 1.153 x 10-2 s-1
Question 16.
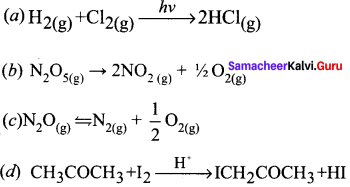
Give the examples for a zero order reaction.
Answer:
Examples for a zero order reaction:
1. Photochemical reaction between H2 and Cl
H2(g) + Cl2(g) \(\underrightarrow { h\nu }\) 2HCI(g)
2. Decomposition of N2O on hot platinum surface
N2 O(g) \(\rightleftharpoons\) N2(g) + \(\frac { 1 }{ 2 }\) O2(g)
3. lodination of acetone in acid medium is zero order with respect to iodine.
CH3COCH3 + I2 \(\underrightarrow { { H }^{ + } } \) ICH2COCH3 + HI
Rate k [CH3COCI3] [H+]
Question 17.
Explain pseudo first order reaction with an example.
Answer:
A second order reaction can be altered to a first order reaction by taking one of the reactant in large excess, such reaction is called pseudo first order reaction. Let us consider the acid hydrolysis of an ester,
CH3COOCH3(aq) +H2 O(1) \(\underrightarrow { { H }^{ + } } \) CH3COOH(aq) + CH3OH(aq)
Rate = k [CH3COOCH3] [H2O]
If the reaction is carried out with the large excess of water, there is no significant change in the concentration of water during hydrolysis. i.e., concentration of water remains almost a constant. Now we can define k [H2O] = k
∴ The above rate equation becomes
Rate k [CHCOOCH] Thus it follows first order kinetics.
Question 18.
Identify the order for the following reactions
- Rusting of Iron
- Radioactive disintegration of 92U23
- 2 A+ B → products; rate = k [A]1/2 [B]2
Answer:
1.
Theoritically order value may be more than one but practically one.
2. All radioactive disintegrations are first order reactions
3. 2A + 3B → products:
rate = k[A]1/2 [B]2
Order = \(\frac { 1 }{ 2 }\) + 2 = \(\frac { 5 }{ 2 }\) = 2.5
Question 19.
A gas phase reaction has energy of activation 200 kJ mol-1. If the frequency factor of the reaction is 1.6 x 1013 s-1. Calculate the rate constant at 600 K. (e-40.09 = 3.8 x I0-18 )
Solution:
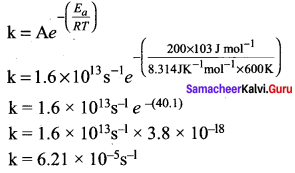
Question 20.
For the reaction 2x +y → L find the rate law from the following data.
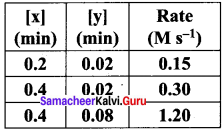
Answer:
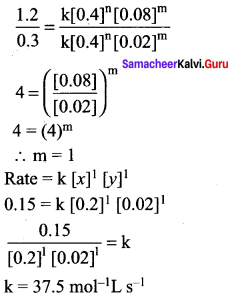
Rate = k [x]n [y]m
0.15 = k [0.2]n [0.02]m ……………..(1)
0.30 = k [0.4]n [0.02]m ……………… (2)
1.20 = k [0.4]n [0.08]m ……………… (3)
Dividing Eq(3) by Eq (2) we get
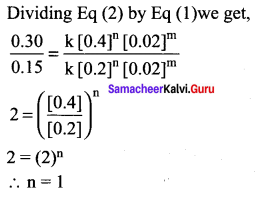
Question 21.
How do concentrations of the reactant influence the rate of reaction?
Answer:
The rate of a reaction increases with the increase in the concentration of the reactants. The effect of concentration is explained on the basis of collision theory of reaction rates.
According to this theory, the rate of a reaction depends upon the number of collisions between the reacting molecules. Higher the concentration, greater is the possibility for collision and hence the rate.
Question 22.
How do nature of the reactant influence rate of reaction?
Answer:
Nature and state of the reactant:
We know that a chemical reaction involves breaking of certain existing bonds of the reactant and forming new bonds which lead to the product.
The net energy involved in this process is dependent on the nature of the reactant and hence the rates arc different for different reactants. Let us compare the following two reactions that we carried out in volumetric analysis.
- Redox reaction between ferrous ammonium sulphate (FAS) and KMnO4
- Redox reaction between oxalic acid and KMnO4
The oxidation of oxalate ion by KMnO4 is relatively slow compared to the reaction between KMnO4 and Fe . In fact heating is required for the reaction between KMnO4 and Oxalate ion and is carried out at around 60°C. The physical state of the reactant also plays an important role to influence the rate of reactions. Gas phase reactions are faster as compared to the reactions involving solid or liquid reactants.

For example, reaction of sodium metal with iodine vapours is faster than the reaction between solid sodium and solid iodine. Let us consider another example that we carried out in inorganic qualitative analysis of lead salts.
If we mix the aqueous solution of colorless potassium iodide with the colorless solution of lead nitrate, precipitation of yellow lead iodide take place instantancously, whereas if we mix the solid lead nitrate with solid potassium iodide, yellow coloration will appear slowly.
Question 23.
The rate constant for a first order reaction is 1.54 x 10 s-1. Calculate its half life time.
Answer:
We know that, t, 0.693 k
t1/2 = 0.693/1.54 x 1o-3 = 450 s
Question 24.
The half life of the homogeneous gaseous reaction SO2CI2 → SO2 + Cl2 which obeys first order kinetics Is 8.0 minutes. How long will it take for the concentration of SO2Cl2 to be reduced to 1% of the initial value?
Answer:
We know that, k = 0.693/ t1/2
k = 0.693/8.0 minutes = 0.087 minutes -1
For a first order reaction,
k = \(\frac { 2.303 }{ k }\) log \(\left( \frac { \left[ { A }_{ 0 } \right] }{ \left[ A \right] } \right)\)
t = \(\frac { 2.303 }{ 0.087{ min }^{ -1 } }\) log\(\frac { 100 }{ 1 }\)
t = 52.93 mm
Question 25.
The time for half change in a first order decomposition of a substance A is 60 seconds. Calculate the rate constant. How much of A will be left after 180 seconds?
Answer:
1. Order of a reaction = 1
t1/2 = 60
seconds, k = ?
k = \(\frac { 2.303 }{ 60 }\)
We know that, k = \(\frac { 2.303 }{ { t }_{ 1/2 } }\)
k = \(\frac { 2.303 }{ 60 }\) = 0.01155 s-1
2. [A0] = 100%
t = 180 s
k = 0.01155 seconds-1
[A] = ?
For the first order reaction k = \(\frac { 2.303 }{ 60 }\) log \(\left( \frac { \left[ { A }_{ 0 } \right] }{ \left[ A \right] } \right)\)
0.9207 = log 100 – log [A]
log [A] = log 100 – 0.9207
log [A] = 2 – 0.9207
log[A] = 1.0973
[A] = antilog of (1.0973)
[A] = l2.5%
Question 26.
A zero order reaction is 20% complete in 20 minutes. Calculate the value of the rate constant. In what time will the reaction be 80% complete?
Answer:
1. A = 100%, x = 20%, Therefore, a – x =100 – 20 = 80
For the zero order reaction k= \((\frac { x }{ t })\) ⇒
k = \((\frac { 20 }{ 20 })\) = 1
Rate constant for a reaction = 1
2. To calculate the time for 80% of completion
k = 1, a = l00, x = 80%, t = ?
Therefore, t = \((\frac { x }{ k })\) = \((\frac { 80 }{ 1 })\) = 80 min
Question 27.
The activation energy of a reaction is 225 k cal mol-1 and the value of rate constant at 40°C is 1.8 x 10-5 s-1. Calculate the frequency factor, A. Here, we arc given that
Answer:
Ea = 22.5 kcal mol-1 = 22500 cal mol-1
T = 40°C = 40 + 273 = 313 K
k = 1.8 x 10-5 sec-1
Substituting the values in the equation
log A = log k + \(\left( \frac { { E }_{ a } }{ 2.303RT } \right)\)
log A = log (l .8 x 10-5) + \(\left( \frac { 22500 }{ 2.303\times 1.987\times 313 } \right)\)
log A = log (l.8) – 5 + (15.7089)
log A = (10.9642)
A = antilog ( 10.9642)
A = 9.208 x 1010 collisions s-1
Question 28.
Benzene diazonium chloride in aqueous solution decomposes according to the equation
C6H5N2CI C6H5CI + N2. Starting with an initial concentration of 10 g L-1 volume of N2. gas obtained at 50°C at different intervals of time was found to be as under:

Show that the above reaction follows the first order kinetics. What is the value of the rate constant ?
Solution:
For a first order reaction
k = \(\frac { 2.303 }{ t }\) log \(\frac { a }{ (a-x) }\)
k = \(\frac { 2.303 }{ t }\) log \(\frac { { V }_{ \infty } }{ { V }_{ \infty }-{ V }_{ t } }\)
In this case, V∞ = 58.3 ml
The value of k at different time can be calculated as follows:
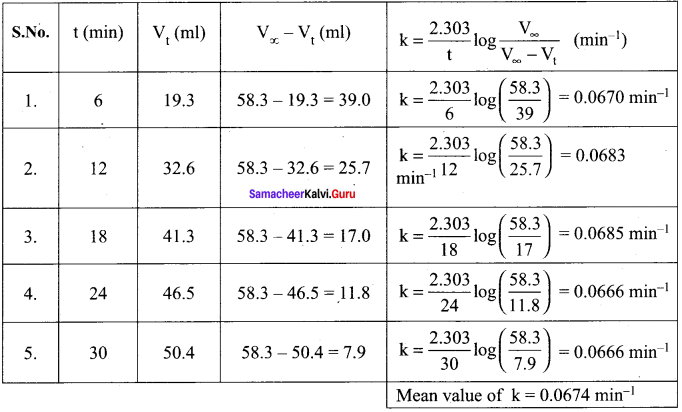
Since the value of k comes out to be nearly constant, the given reaction is of the first order. The mean value of k = 0.0674 min-1
Question 29.
From the following data, show that the decomposition of hydrogen peroxide is a reaction of the first order:

Where t is the volume of standard KMnO4 solution required for titrating the same volume of the reaction mixture.
Solution:
Volume of KMnO4 solution used Amount of H2O2 present. Hence if the given reaction is of the first order, it must obey the equation
k = \(\frac { 2.303 }{ t }\) log \(\frac { a }{ (a-x) }\)
k = \(\frac { 2.303 }{ t }\) log \(\frac { { V }_{ 0 } }{ { V }_{ t } }\)
In this case,V0 = 46.1 ml
The value of k at each instant can be calculated as follows:
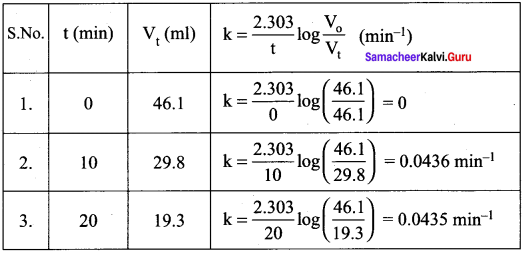
Thus, the value of k comes out to be nearly constant. Hence it is a reaction of the first order.
Question 30.
A first order reaction is 40% complete in 50 minutes. Calculate the value of the rate constant. in what time will the reaction be 80% complete?
Answer:
1. For the first order reaction k = \(\frac { 2.303 }{ t }\) log \(\frac { a }{ (a-x) }\)
Assume, a = 100 %, x = 40%, t = 50 minutes
Therefore, a – x = 100 – 40 = 60
k = (2.303/50) log (100/60)
k = 0.010216 min-1
Hence the value of the rate constant is 0.010216 min-1
2. t = ?, when x = 8O%
Therefore, a – x = 100 – 80 = 20
From above, k = 0.0102 16 min-1
t = (2.303 / 0.010216) log (100 / 20)
t = 157.58 min
The time at which the reaction will be 80% complete is 157.58 min.
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements Evaluate yourself
Question 1.
Write the rate expression for the following reactions, assuming them as elementary reactions.
- 3A + 5B2 → 4CD
- X2 + Y2 → 2XY
Answer:
1. 3A + 5B2 → 4CD
Rate = – \(\frac { 1 }{ 3 }\) \(\frac { \triangle [A] }{ dt }\)
= – \(\frac { 1 }{ 5 }\) \(\frac { \triangle [{ B }_{ 2 }] }{ dt }\)
= + \(\frac { 1 }{ 4 }\) \(\frac { \triangle [CD] }{ dt }\)
2. X2 + Y2 → 2XY
Rate = – \(\frac { \triangle [{ X }_{ 2 }] }{ dt }\)
= + \(\frac { 1 }{ 2 }\) \( [latex]\frac { \triangle [{ XY }_{ 2 }] }{ dt }\)
Question 2.
Consider the decomposition of N2O5(g) to form NO2(g) and O2(g). At a particular instant N2O5 disappears at a rate of 2.5 x 10-2 mol dm-3 s-1. At what rates are NO2 and O2 formed? What is the rate of the reaction?
Solution:
2N2O5(g) → 4NO2(g) + O2(g)
from the stoichiometry of the reaction.
– \(\frac { 1 }{ 2 }\) \(\frac { d[{ N }_{ 2 }{ O }_{ 5 }] }{ dt }\)
= \(\frac { 1 }{ 4 }\) \(\frac { d[{ N }{ O }_{ 2 }] }{ dt }\)
= –\(\frac { d[{ N }{ O }_{ 2 }] }{ dt }\)
= 2 –\(\frac { d[{ N }_{ 2 }{ O }_{ 5 }] }{ dt }\)
Rate of disappearance of N2O5 is 2.5 x 10-2 mol dm-3 s-1
∴ The rate of formation of NO2 at this temperature is 2 x 2.5 x 10-2 = 5 x 10-2 mol dm-3 s-1.
– \(\frac { 1 }{ 2 }\) \(\frac { d[{ N }_{ 2 }{ O }_{ 5 }] }{ dt }\)
= – \(\frac { d[{ O }_{ 2 }] }{ dt }\)
∴ \(\frac { d[{ O }_{ 2 }] }{ dt }\) = \(\frac { 1 }{ 2 }\) x 2.5 x 10-2 mol dm-3 s-1
= 1.25 x 10-2 mol dm-3 s-1
Question 3.
For a reaction, X + Y → Product quadrupling [x], increases the rate by a factor of 8. Quailrupling both [x] and [y] increases the rate by a factor of 16. Find the order of the reaction with respect to x and y. what is the overall order of the reaction?
Solution:
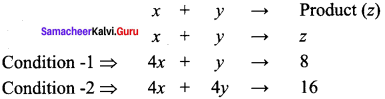
z = k[x]m [y]n …………(1)
8z = k[x]m [y]n …………(2)
16z = k[x]m [y]n ……………(3)
Dividing Eq (2) by Eq (1) we get,
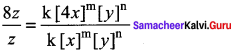
8 = 4m ⇒23 = (22)m ⇒ 2 = 22m
2m = 3
m = 3/2
1.5 order with respect to x.
Dividing Eq (3) by Eq (1) we get,
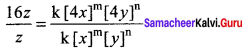
16 = 4m. 4n
16 = 42. 4n
\(\frac { 16 }{ 16 }\) = 4n
1 = 4n
∴ n = 0 [Zero order with respect to y]
Overall order of the reaction.
k [x]m [y]n
k [x]1.5 [y]0
Order (1.5+0) = 1.5
Question 4.
Find the individual and overall order of the following reaction using the given data.
2NO(g) + Cl2 (g) → 2NOCI(g)
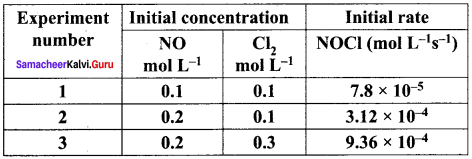
Solution:
Rate = k [NO]m [CI2]
For experiment 1, the rate law is,
Rate1 = k [NO]m [CI2]n
7.8 x 10-5 k[0.1]m [0.1]n ………………(1)
For experiment 2, the rate law is.
Rate2 = k [NO]m [CI2]n
3.12 x 10-4 = k[O.2]m [0.1]n ……………….(2)
For experiment 3, the rate law is,
Rate3 = k [NO]m [CI2]n
9.36 x 10-4 = k [O.2]m [0.3]m ……………(3)
Dividing Eq (2) by Eq (l) we get,
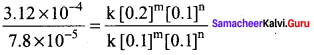
4 = \(\frac { 0.2 }{ 0.1 }\)m
⇒ 22 = 2m
∴m = 2
Therefore the reaction is secondary order with respect to NO.
Dividing Eq (3) by Eq (2) we get,
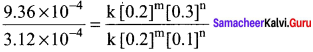
Therefore the reaction is first order with respect to Cl2
The rate law is, Rate = k [NO]2 [Cl2]1
The overall order of the reaction (2 +1) = 3.
Question 5.
In a first order reaction A → products, 60% of the given sample of A decomposes in 40 min. what is the half life of the reaction?
Solution:
k = \(\frac { 2.303 }{ t }\) log \(\frac{\left[\mathrm{A}_{0}\right]}{[\mathrm{A}]}\)
k = \(\frac { 2.303 }{ 40min }\) log \(\frac { 100 }{ (100-60) }\)
k = 0.0575 (0.3979) ⇒ k = 0.02287 min-1
t1/2 = \(\frac { 0.6932 }{ k }\) log \(\frac { 0.6932 }{ 0.02287 }\)
t1/2 = 30.31 min.
Question 6.
The rate constant for a first order reaction is 2.3 x 10-4 s-1. If the initial concentration of the reactant is 0.01 M. what concentration will remain after 1 hour?
Solution:
Rate constant of a first order reaction k = 2.3 x 10-4 s-1
Initial concentration of the reactant [A0] = 0.01 M
Initial concentration ot the reactant [A0] = 0.01 M
Concentration will remain after 1 hour [A] =7
k = \(\frac { 2.303 }{ t }\) log \(\frac{\left[\mathrm{A}_{0}\right]}{[\mathrm{A}]}\)
2.3 x 10-4 = \(\frac { 2.303 }{ 1 hour }\) log \(\frac { [0.01] }{ [A] }\)
\(\frac { 2.3\times { 10 }^{ -4 }\times 1 }{ 2.303 }\) = log [0.01] – log[A]
9.986 x 10-5 = – 2 – log [A]
11.986 x 10-5 = – log [A]
[A] = Antilog (-11.986 x 10-5)
[A] = 0.997 M
Question 7.
Hydrolysis of an ester in an aqueous solution was studied by titrating the liberated carboxylic acid against sodium hydroxide solution. The concentrations of the ester at different time intervals are given below.

Show that, the reaction follows first order kinetics.
Solution:
The value of k at different time can be calculated as follows:
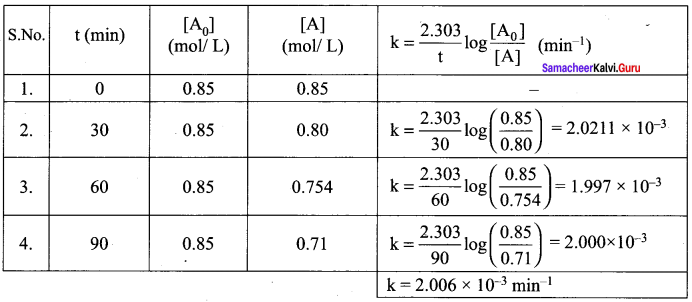
This value shows that reaction follows first order kinetics.
Question 8.
For a first order reaction the rate constant at 500K is 8 x 10-4 s-1. Calculate the frequency factor, if the energy of activation for the reaction is 190 kJ mol-1.
k = 8 x 10-4s
T = 500K
Ea = 190 kJ mol-1 A = ?
According to Arrhenius equation,
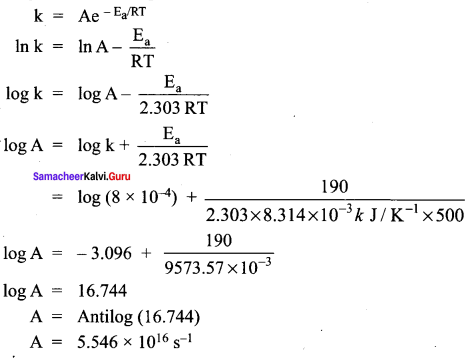
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements Text book Example problems
Question 1.
The oxidation of nitric oxide (NO)
2NO(g) + O2(g) → NO2(g)
Series of experiments are conducted by keeping the concentration of one of the reactants constant and the changing the concentration of the others.

Find out the individual overall order of the reaction.
Solution:
Rate = k [NO]m [O2]n
For experiment 1, the rate law is
Rate2 = k [NO]m [O2]n
19.26 x 10-2 = k[1.3]m [1.1]n ………………(1)
Similarly for experiment 2
Rate2 = k [No]m [O2]n
38.40 x 10-2 = k [1.3]m [2.2]n …………………..(2)
For experiment 3
Rate3 = k [NO]m [O2]n
76.8 x 10-2 = k [2.6]m [1.1]n ……….(3)
Dividing Eq (2) by Eq (1)
\(\frac { 38.40\times { 10 }^{ -2 } }{ 19.26\times { 10 }^{ -2 } }\) = \(\frac { k{ \left[ 1.3 \right] }^{ m }{ \left[ 2.2 \right] }^{ n } }{ k{ \left[ 1.3 \right] }^{ m }{ \left[ 1.1 \right] }^{ n } }\)
2 = \({ \left( \frac { 2.2 }{ 1.1 } \right) }^{ n }\)
2 = 2n
i.e., n= 1
Therefore the reaction is first order with respect to O2
Dividing Eq (3) byEq (1)
\(\frac { 76.8\times { 10 }^{ -2 } }{ 19.26\times { 10 }^{ -2 } }\) = \(\frac { k{ \left[ 2.6 \right] }^{ m }{ \left[ 1.1 \right] }^{ n } }{ k{ \left[ 1.3 \right] }^{ m }{ \left[ 1.1 \right] }^{ n } }\)
4 = \({ \left( \frac { 2.6 }{ 1.3 } \right) }^{ m }\)
4 = 2m
i.e., m = 2
Therefore the reaction is second order with respect to NO
The rate law is Rate1 = k [NO]2 [O2]1
The overall order of the reaction = (2 + 1) = 3
Question 2.
Consider the oxidation of nitric oxide to form NO2
2NO(g) + O2(g) → 2NO2(g)
- Express the rate of the reaction in terms of changes in the concentration of NO, O2 and NO2.
- At a particular instant, when [O2] is decreasing at 0.2 mol L-1 s-1 at what rate is
[NO2] increasing at that instant?
Solution:
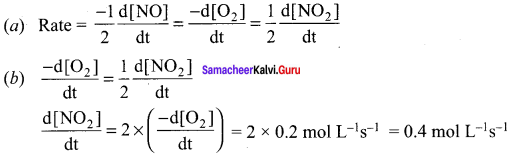
Question 3.
What is the order with respect to each of the reactant and overall order of the following reactions?
1. 5Br–(aq) + BrO3– (aq)+ 6H+(aq) → 3Br2(1) +3H2O(1)
The experimental rate law is
Rate = k [Br–] [BrO3–][H+]2
2. CH3 CHO(g) \(\underrightarrow { \triangle }\) CH4(g) + CO(g)
the experimental rate law is
Rate = k [CH3CHO]3/2
Solution:
1. First order with respect to Br–, first order with respect to BrO3– and second order with respect to H+. Hence the overall order of the reaction is equal to 1+1+2=4
2. Order of the reaction with respect to acetaldehyde is \(\frac { 3 }{ 2 }\)and overall order is also \(\frac { 3 }{ 2 }\)
Question 4.
The rate of the reaction x + 2y → product is 4 x 10-3 mol L-1 s-1, if [x] = [y] = 0.2 M and rate constant at 400K is 2 x 10-2 s-1. what is the overall order of the reaction.
Solution:
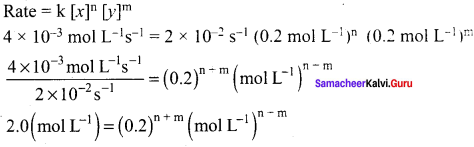
Comparing the powers on both sides, the overall order of the reaction n + m = 1
Question 5.
A first order reaction takes 8 hours for 90% completion. Calculate the time required for 80% completion. (log 5 = 0.6989; log10 = 1)
Solution:
For a first order reaction,
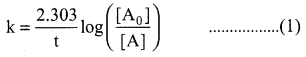
Let [A0] = 100M
When
t = t90%; [A] = 10M (given that t90% = 8 hours)
t = t80%; [A] = 20M
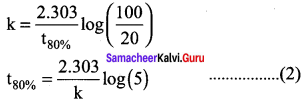
Find the value of k using the given data
k = \(\frac{2.303}{t_{90 \%}}\) log \((\frac { 100 }{ 10 })\)
k = \(\frac { 2.303 }{ 8 hours }\) log 10
k = \(\frac { 2.303 }{ 8 hours }\) (1)
Substitute the value of k in equation (2)
t80% = \(\frac { 2.303 }{ 2.303/8 hours }\) log (5)
t80% = 8 hours x 0.6989
t80% = 5.59 hours
Question 6.
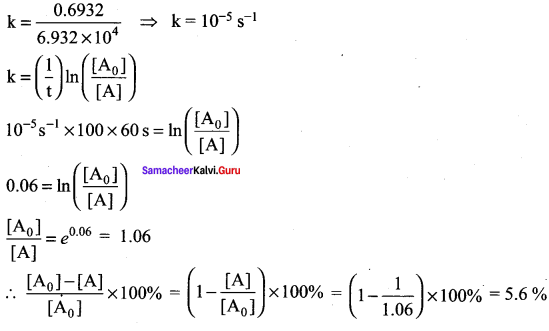
The half life of a first order reaction x → products is 6.932 x 104 s at 500K . What percentage of x would be decomposed on heating at 500K for 100 min. (e0.06 = 1.06)
Solution:
Given t1/2 = 0.6932 x 104 s
To solve: when t = 100 min
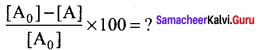
We know that for a first order reaction,t1/2 = \(\frac { 0.6932 }{ k }\)
Question 7.
Show that in case of first order reaction, the time required for 99.9% completion is nearly ten times the time required for half completion of the reaction.
Solution:
Let [A0] = 100
When t = t99.9%; [A] = (100 – 99.9) = 0.1
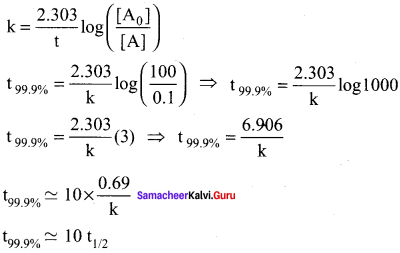
Question 8.
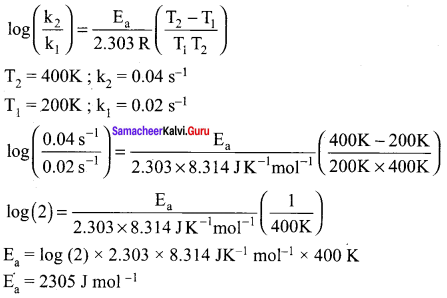
The rate constant of a reaction at 400 and 200K are 0.04 and 0.02 s-1 respectively. Calculate the value of activation energy.
Answer:
According to Arrhenius equation.
Question 9.
Rate constant k of a reaction varies with temperature T according to the following Arrhenius equation. log k = log A \(\frac { { E }_{ a } }{ 2.303R }\) \(\frac { 1 }{ T }\).
Where E is the activation energy. When a graph is plotted for log k Vs \(\frac { 1 }{ T }\) a straight line with a slope of 4000K is obtained. Calculate the activation energy.
Solution:
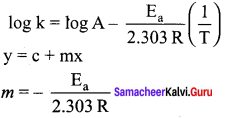
Ea = – 2.303 Rm
Ea = – 2.303 x 8.314 JK-1 mol-1 x (-4000k)
Ea = 76,589 J mol-1
Ea = 76,589 kJ mol-1
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements Additional Questions
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements 1 Mark Questions and Answers
I. Choose the correct answer.
Question 1.
Which one of the following is a slow reaction?
(a) Rusting of iron
(b) Combustion of carbon
(c) Reaction between BaCl2 and dil. H2SO4
(d) Reaction between acidified K2Cr2O7 with NaCl.
Answer:
(a) Rusting of iron
Question 2.
Which one of the following is the unit of rate of reaction?
(a) s-1
(b) mol s-1
(c) mol L-1 s-1
(d) mol L s
Answer:
(c) mol L-1 s-1
Question 3.
For a gas phase reaction, the unit of reaction rate is ……………
(a) s-1
(b) atm s-1
(c) mol L-1 s-1
(d) mol-1 L-1 s-1
Answer:
(b) atm s-1
Question 4.
For the reaction A → 2B, the rate of the reaction is ………….
(a) +\(\frac { d[B] }{ dt }\) = 2 – \(\frac { d[A] }{ dt }\)
(b) +\(\frac { d[A] }{ dt }\) = \(\frac { 1 }{ 2 }\) \(\frac { d[B] }{ dt }\)
(c) Rate = \(\frac { 1 }{ 2 }\) = \(\frac { d[A] }{ dt }\)
(d) Rate = 2\(\frac { d[B] }{ dt }\)
Answer:
(a) +\(\frac { d[B] }{ dt }\) = 2 – \(\frac { d[A] }{ dt }\)
Question 5.
Consider the following statement.
(i) In ionisation of cyclopropane, if the concentration of cyclopropane is reduced half, the rate increases twice.
(ii) The rate of the reaction depends upon the concentration of the reactant.
(iii) Order values must be determined experimentally.
Which of the above statement (s) is / are not correct?
(a) (i) only
(b) (ii) and (iii)
(c) (iii) only
(d) (ii) only
Answer:
(a) (i) only
Question 6.
In the reaction 2NO(g) + O2(g) → 2NO2(g) the order of the reaction with respect to NO is…………
(a) first order
(b) second order
(c) third order
(d) zero order
Answer:
(b) second order
Question 7.
In the reaction 2NO(g) + O2(g) → 2NO2(g). the order of the reaction with respect to O2is …….
(a) zero order
(b) first order
(c) second order
(d) third order
Answer:
(b) first order
Question 8.
The overall order of the reaction 2NO(g) + O2(g) → 2NO2(g) is …………….
(a) 2
(b) 1
(c) 3
(d) 0
Answer:
(c) 3
Question 9.
Consider the following statements.
(i) Rate of the reaction does not depend on the initial concentration of the reactants.
(ii) Rate constant of the reaction depends on the initial concentration of reactants.
(iii) Rate constant of the reaction is equal to the rate of the reaction, when the concentration of each of the reactants is unity.
Which of the above statement(s) is / are not correct?
(a) (i) only
(b) (ii) only
(c) (i) and (ii)
(d) (iii) only
Answer:
(a) (iii) only
Question 10.
The overall molecularity of the reaction 2H2 O2(aq) \(\underrightarrow { { I }^{ – } }\) 2H2O1 + O2(g) is …………
(a) unimolecular
(b) bimolecular
(c) termolecular
(d) pentamolecular
Answer:
(b) bimolecular
Question 11.
Which of the following is the order of decomposition of hydrogen peroxide catalysed by I– ………….
(a) First order
(b) Second order
(c) Zero order
(a) Third order
Answer:
(a) First order
Question 12.
Consider the following statements.
(i) order cannot be zero.
(ii) Molecularity can be zero (or) fractional (or) integer.
(iii) order can be determined only by experiment.
Which of the above statement(s) is / are not correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i) and (ii)
Answer:
(c) (iii) only
Question 13.
The overall order of the reaction 5Br– + BrO3– + 6H+ is ……..
(a) 4
(b) 3/2
(c) 12
(d) 1
Answer:
(a) 4
Question 14.
Which one of the following reaction is a fractional order reaction?
(a) 2NO +O2 → 2NO2
(b) CH3CHO(g) → CH4(g) + CO(g)
(c) 2H2O2 → 2H2)O(1) + O2(g)
(d) H2 + Br2 → 2HBr
Answer:
(b) CH3CHO(g) → CH4(g) + CO(g)
Question 15.
The order of decomposition of acetaldehyde is …………….
(a) 1
(b) 1.5
(c) 2
(d) 5/2
Answer:
(b) 1.5
Question 16.
Which one of the following is the unit of rate constant for a first order reaction?
(a) mol-1 L s-1
(b) mol L-1 s-1
(c) s-1
(d) mol L S
Answer:
(c) s-1
Question 17.
Which one of the following is an example for first order reaction?
(a) 2NO(g)+ O2(g) → 2NO2(g)
(b) CH3CHO(g) → CH4(g) + CO(g)
(c) SO2 Cl2(1) → SO2(g) + Cl2(g)
(d) 2HBr → H2 + Br2
Answer:
(c) SO2 Cl2(1) → SO2(g) + Cl2(g)
Question 18.
Which one of the following is not an example for first order reaction?
(a) N2O5(g) → 2NO2(g) \(\frac { 1 }{ 2 }\) O2(g)
(b) SO2Cl2(1) → SO2(g) + Cl2(g)
(e) H2O2(aq) → H2O1\(\frac { 1 }{ 2 }\)O2(g)
(d) CH3CHO(g) → CH4(g) + CO(g)
Answer:
(d) CH3CHO(g) → CH4(g) + CO(g)
Question 19.
What is the order of isomerisation of cyclopropane to propene?
(a) 1.5
(b) 3/2
(c) 5/2
(d) 1
Answer:
(d) 1
Question 20.
Which one of the following is an example of pseudo first order reaction?
(a) CH3CHO4(g) → CH4(g) + CO(g)
(b) 2H2O2(aq) → H2O(1) +O2(g)
(c) CH3COOCH3(aq) + H2O(1) \(\underrightarrow { { H }^{ + } }\) CH3COOH(aq) + CH3OH(aq)
(d) Isomerisation of cyclo propane to propene
Answer:
(c) CH3COOCH3(aq) + H2O(1) \(\underrightarrow { { H }^{ + } }\) CH3COOH(aq) + CH3OH(aq)
Question 21.
Which one of the following is called pseudo first order reaction?
(a) Decomposition of acetaldehyde
(b) Acid hydrolysis of an ester
(c) Isomerisation of cyclopropane to propene
(d) Decomposition of hydrogen peroxide
Answer:
(b) Acid hydrolysis of an ester
Question 22.
Which of the following is an example of zero order reaction?
(a) lodination of acetone in acid medium
(b) Hydrolysis of an ester in acid medium
(c) Decomposition of acetaldehyde
(d) Isomerisation of cyclopropane to propene
Answer:
(a) lodination of acetone in acid medium
Question 23.
Which one of the follow is not zero order reaction?
(a) H2(g) + Cl2(g) \(\underrightarrow { h\nu }\) 2HCI(g)
(b) N2O(g) \(\rightleftharpoons\) N2(g) + \(\frac { 1 }{ 2 }\) O2(g)
(c) CH3CHO(g) → CH4(g)+ CO(g)
(d) CH3COCH3 + I2 \(\underrightarrow { { H }^{ + } }\) CH2COCH3 + HI
Answer:
(c) CH3CHO(g) → CH4(g)+ CO(g)
Question 24.
Consider the following statements.
(i) For a first order reaction, half life period is independent of initial concentration.
(ii) Photo chemical reaction between H2 and Cl2 is a zero order reaction
(iii) Acid hydrolysis of an ester is a second order reaction
Which of the above statement is/are correct?
(a) (i) only
(b) (iii) only
(c) (i) & (ii)
(d) (ii) & (iii)
Answer:
(c) (i) & (ii)
Question 25.
The formula of half life for an nth order reaction involving reactant A and n \(\neq\) 1 is
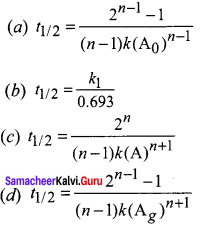
Answer:
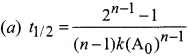
Question 26.
The half life period of first order reaction is 10 seconds. What is the time required for 99.9% completion of that reaction?
(a) 20 seconds
(b) 1000 seconds
(c) 100 seconds
(d) 999 seconds
Answer:
(c) loo seconds
Hint:
10 x t1/2 = t99.9%
∴ t99.9% = 10 x 10 sec = 100 sec
Question 27.
Which one of the following is known as arrhenius equation?
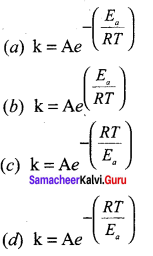
Answer:
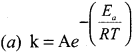
Question 28.
Which one of the following does not affect the rate of the reaction?
(a) Nature of the reactant
(b) Concentration of the reactants
(c) Surface area and temperature
(d) pressure
Answer:
(d) pressure
Question 29.
Consider the following statements.
(i) Higher the concentration, slower is the possibility for collision and rate also slower
(ii) Increase in surface area of reactant leads to more collisions per litre per second
(iii) Gas phas reactions are slower as compared to solid or liquid reactants
Which of the above statement is/are not correct?
(a) (ii)
(b) (i) & (iii)
(c) (ii) & (iii)
(d) (i) & (ii)
Answer:
(b) (i) & (iii)
Question 30.
Which of the following reaction take place at a faster rate?
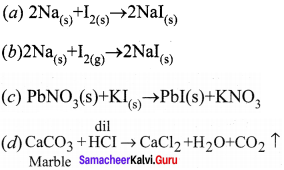
Answer:
![]()
Question 31.
Which one of the following graph is not correct ………..
Answer:
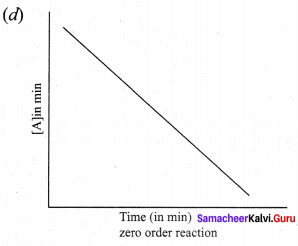
Question 32.
The half life of paracetamol with in the body is ………
(a) 2 hours
(b) 2.5 hours
(c) 6 hours
(d) 10 hours
Answer:
(b) 2.5 hours
Question 33.
What is the order of radioactive decay?
(a) first order
(b) zero order
(c) second order
(d) third order
Answer:
(a) first order
Question 34.
t1/2 of the reaction increases with increase in initial concentration of the reaction means the order of the reaction will be …………
(a) first order
(b) zero order
(c) second order
(d) third order
Answer:
(b) zero order
Question 35.
The reaction rate that does not decrease with time is …………
(a) pseudo first order reaction
(b) first order reaction
(c) zero order reaction
(d) second order reaction
Answer:
(c) zero order reaction
Question 36.
The rate of the reaction X → Y becomes 8 times when the concentration of the reactant ‘X’ is doubled. The rate law of the reaction is ………
(a) – \(\frac { d[x] }{ dt }\) = k[X]2
(b) – \(\frac { d[x] }{ dt }\) = k[X]3
(c) – \(\frac { d[x] }{ dt }\) = k[X]4
(d) – \(\frac { d[x] }{ dt }\) = k[X]8
Answer:
(b) – \(\frac { d[x] }{ dt }\) = k[X]3
Question 37.
The decomposition of ammonia gas on platinum surface has a rate constant k = 2.5 x 10-4 mol L-1 s-1 What is the order of the reaction?
(a) first order
(b) second order
(c) third order
(d) zero order
Answer:
(d) zero order
Question 38.
A reaction is 50% completed in 2 hours and 75% completed in 4 hours. Then the order of the reaction is ………….
(a) first order
(b) zero order
(c) second order
(d) third order
Answer:
(a) first order
Answer:
(a) first order
Question 39.
What is the rate equation for the reaction A + B → C has zero order?
(a) Rate = k
(b) Rate = k [A]
(c) Rate = k [A]. [B]
(a) Rate = k. \(\frac { 1 }{ [c] }\)
Answer:
(c) Rate = k [A]. [B]
Question 40.
How does the value of rate constant vary with reactant concentration?
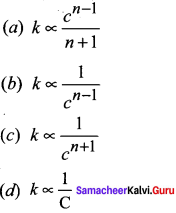
Answer:
![]()
Question 41.
Identify the reaction order if the unit of rate constant is s-1 ……….
(a) zero order reaction
(b) second order reaction
(c) first order reaction
(d) third order reaction
Answer:
(c) first order reaction
Question 42.
What is unit of zero order reaction?
(a) s-1
(b) mol-1 L-1 s-1
(c) mol L-1 s-1
(d) mol L s-1
Answer:
(c) mol L-1 s-1
Question 43.
Which of the following factor affect the rate of the reaction’?
(a) volume
(b) pressure
(c) cone
(d) all the above
Answer:
(c) cone
Question 44.
Acid hydrolysis of an ester is an example of ………
(a) zero order reaction
(b) Pseudo first order reaction
(c) second order reaction
(d) first order reaction
Answer:
(b) Pseudo first order reaction
Question 45.
Polymerisation reactions follows ………………. order kinetics.
(a) fractional
(b) first
(c) zero
(d) Pseudo first
Answer:
(a) fractional
Question 46.
Activation energy of a chemical reaction can be determined by ……….
(a) changing concentration of the reactants
(b) Evaluating rate constants at standard temperature
(c) Evaluating rate constants at two different temperature
(d) Evaluating reIocities of reaction at two different temperature
Answer:
(c) Evaluating rate constants at two different temperature
Question 47.
Which of the following is the fastest reaction?
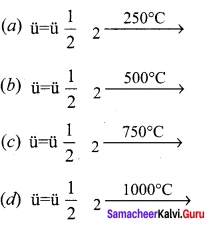
Answer:
![]()
Question 48.
Half life period of a reaction is found to be inversely proportional to the cube of its initial concentration. The order of the reaction is ………
(a) 2
(b) 5
(c) 3
(d) 4
Answer:
(d) 4
Question 49.
A large increase in the rate of a reaction for a rise in temperature is due to ………
(a) the decrease in the number of collisions
(b) increase in the number of activated molecules
(c) the shortening of mean free path
(d) the lowering of activation energy
Answer:
(b) increase in the number of activated molecules
Question 50.
The minimum energy of a molecule would possess in order to enter into a fruitful collision is known as …………..
(a) Reaction energy
(b) collision energy
(c) Activation energy
(d) Threshold energy
Answer:
(a) Threshold energy
II. Fill in the blanks.
- The unit of the rate of a reaction is ………..
- For a ……….. reaction, the unit of the reaction rate is atm s
- An elementary step is characterised by its ………..
- The total number of reactant species involved in an elementary step is called ………..
- The sum of powers of concentration terms involved in the experimentally determined rate law is called ………..
- The overall order of decomposition of acetaldehyde to methane and carbon monoxide is ………..
- A second order reaction can be altered to a first order reaction by taking one of the reactant in large excess, it is called ………..
- A reaction in which rate is independent of the concentration of the reactant over a wide range of concentration is called ………..
- All radioactive disentegration reactions follow ……….. kinetics.
- For a first order reaction, half life does not depend on ………..
- Half life period of zero order reaction is ……….. proportional to initial concentration of the reactant.
- Half life period ……….. reaction is directly proportional to initial concentration of the reactant.
- ……….. was proposed by Max Trautz and William lewis.
- Collision theory was proposed by ……….. in 1916 and in ……….. 1918.
- For a gas at room temperature (298 K) and I atm, each molecule undergoes approximately ……….. per second.
- In order to react, the collidng molecules must possess a minimum energy called ………..
- Generally the reaction rate tends to double when the temperature is increased by ………..
- The number of collisions of reactant molecules per second is known as ………..
- Heating is required for the reaction between KMnO4 and oxalate ion and is carried out at around ………..
- ……….. reactions are faster as compared to reactions involving solid or liquid reactants.
- The rate of the reaction ……….. with the increase in the concentration of the reactants.
- Higher the concentration of reactants greater is the possibility of and hence the ………..
- In the presence of catalyst the energy of activation is ……….. and hence greater number of molecules change over to products there by increasing the rate of the reaction.
- Bio availability of drugs within the body and this branch of study is called ………..
- ……….. has a half life of 2.5 hours within the body.
- The change in concentration of species per unit time gives the ……….. of the reaction.
- The rate constant is equal to the rate of the reaction when concentration of reactants is ………..
- Increase in surface area of reactant leads to more collisions per litre per second and hence the rate of the reaction is ………..
- Acid hydroJysis of an ester is an example of ………..
- Molecularity of a chemical reaction will never be equal to ………..
Answer:
- moI L-1 s-1
- gas phase
- molecularity
- Molecularity
- order
- 3/2 or 1.5
- Pseudo first order reaction
- Zero order reaction
- First order
- initial concentration
- directly
- zero order
- Collision theory
- Max Trautz, William lewis
- 10 collisions
- Activation energy
- 10°C
- Frequency factor (A)
- 60°C
- Gas phase
- increases
- collisions, rate
- lowered
- Pharmaco kinetics
- Paracetamol
- rate
- unity
- increased
- Pseudo first order reaction
- zero
III. Match the following
Match the list I and II using the code given below the list.
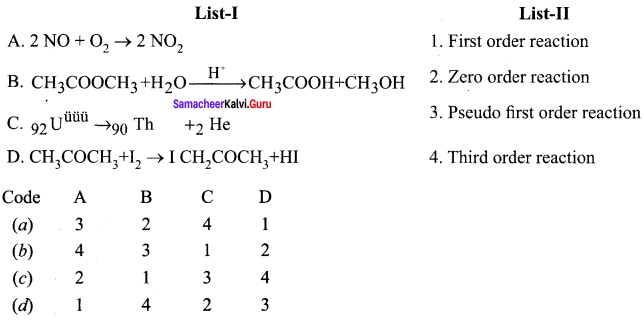
Quetion 1.
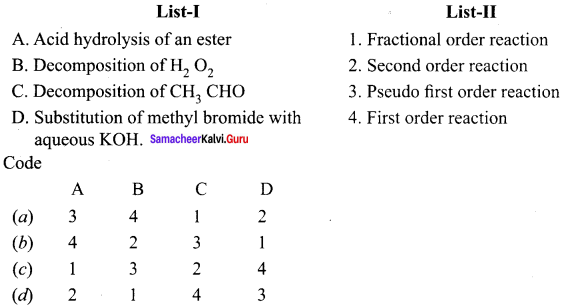
Answer:
(a) 3, 4, 1, 2
Question 2.
Answer:
(a) 3, 4, 1, 2
Question 3.
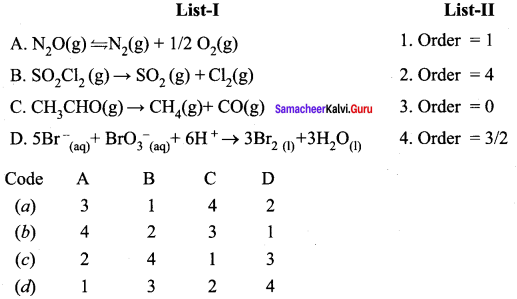
Answer:
(a) 3, 1, 4, 2
Question 4.
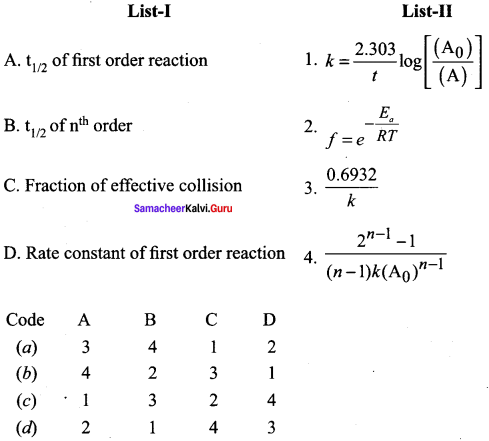
Answer:
(a) 3, 4, 2, 1
IV. Assertion and Reason
Question 1.
Assertion (A): Decomposition of hydrogen peroxide catalysed by I– is a bimolecular first order reaction.
Reason (R): The above reaction take place in two steps, step 1 involves both H2O2 and I and so it is bimolecular but order is determined experimentally as 1.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R arc correct and R is the correct explanation of A
Question 2.
Assertion (A): ![]() The overall order of the reaction is equal to 4.
The overall order of the reaction is equal to 4.
Reason (R): The experimental rate law is.
Rate = K [Br–] [BrO3–] [H+]2.
So 1 + 1 + 2 = 4 order value is 4.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation olA.
Question 3.
Assertion (A): The rate of a reaction increases with the increase in the concentration of the reactants.
Reason (R): The rate of the reaction depends upon the number of collisions between the reacting molecules. Higher the concentration, greater is the possibility for collision and hence the rate.
(a) Both A and R are correct and R is the correct explanation of A,
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 4.
Assertion (A): Powdered calcium carbonate reacts much faster with dilute
HCL than with the same mass of CaCO3 as marble.
Reason (R): For a given mass of a reactant, when the particle size decreases, surface area increases. Increase in surface area of the reactant leads to more collisions per litre per second and hence the rate of the reaction also increases.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R arc correct but R is not correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 5.
Assertion (A): Catalyst presence increases the rate of the reaction
Reason (R): In the presence of a catalyst, energy of activation is lowered and hence greater number of molecules can across the energy harrier and change over to products thereby increasing the rate of the reaction.
(a) Both A and R are correct but R is not correct explanation of A
(b) Both A and R are correct and R is the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(b) Both A and R are correct and R is the correct explanation of A
Question 6.
Assertion (A): Doctors adviced to take paracetamol once in 6 hours during fever and body pain
Reason (R): Paracetarnol has a half life of 2.5 hours within the body. After 10 hours (4 half lives) only 6.25% of drug remains. Based on this, doctors adviced to take it once in 6 hours.
(a) Both A and R are wrong
(b) A is correct but R is wrong
(c) A and R are correct and R is the correct explanation of A
(d) A and R are correct but R is not correct explanation of A
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 7.
Assertion (A): Order of the reaction can be zero or fractional
Reason (R): We cannot determine order from balanced chemical equation
(a) Both A and R are correct but R is not correct explanation of A.
(b) Both A and R are correct and R is the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is not correct explanation of A
Question 8.
Assertion (A): If the activation enery of a reaction is zero, temperature will have no effect on the rate constant
Reason (R): Lower the activation energy, faster is the reaction.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(b) Both A and R are correct but R is not correct explanation of A
V. Find the odd one out
Question 1.
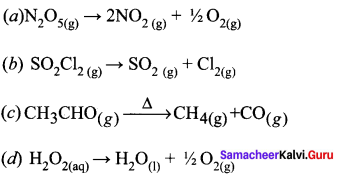
Answer:
![]()
Hint: It is a fractional order reaction with order value 3/2 where as others are first order reaction.
Question 2.
Answer:
![]()
Hint: It is first order reaction whereas others are zero order reaction.
Question 3.
(a) Decomposition of dinitrogen pentoxide
(b) Iodination of acetone in acid medium
(c) Decomposition of N20 on hot Pt surface
(d) photochemical reaction between H2 and CI2
Answer:
(a) Decomposition of dinitrogen pentoxide
Hint: It is a first order reaction whereas others are zero order reactions
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements 2 Mark Questions and Answers
Question 1.
Define rate of the reaction. Give the unit of rate.
Answer:
1. In a chemical reaction, the change in the concentration of the species involved in a chemical reaction per unit time gives the rate of a reaction.
A → B
Rate = –\(\frac { \triangle [A] }{ dt }\) (or) \(\frac { \triangle [B] }{ dt }\)
2.
![]()
= mol L-1 s-1
Question 2.
Define molecularity of a reaction.
Answer:
Molecularity of a reaction is the total number of reactant species that are involved in an elementary step.
Question 3.
Define order of a chemical reaction.
Answer:
Order of a chemical reaction is the sum of powers of concentration terms involved in the experimentally determined rate law.
Question 4.
Define Half life period.
Answer:
The half life ola reaction is defined as the time required for the reactant concentration to reach one half its initial value.
Question 5.
Mention the factors affecting the reaction rate.
Answer:
The rate of the reaction is affected by the following factors.
- Nature and state of the reactant
- Concentration of the reactant
- Surface area of the reactant
- Temperature of the reaction
- Presence of a catalyst
Question 6.
How is surface area of the reactant affect the rate of the reaction?
Answer:
- In heterogeneous reactions, the surface area of the solid reactants play an important role in deciding the rate.
- For a given mass of a reactant, when the particle size decreases surface area increases. Increase in surface area of reactant leads to more collisions per litre per second and hence the rate of reaction is increased.
- For example, powdered calcium carbonate reacts much faster with dilute HCl than with the same mass of CaCO3 as marble.
Question 7.
Paracetamol is prescribed to take once in 6 hours. Justify this statement.
Answer:
1. Paracetamol is a well known antipyretic and analgesic that is prescribed in cases of fever and body pain.
2. Paracetamol has a half life of 2.5 hours within the body. (Le) the plasma concentration of the drug is halved after 2.5 hours. So after 10 hours (4 half lives), only 6.25% of drug remains. Based on this, the dosage and frequency will be decided.
3. In the case of paracetamol, it is usually prescribed to take once in 6 hours.
Question 8.
For a reaction, A + B → product; the rate law is given by r = k[A]1/2 [B]2. What is the order of the reaction?
Answer:
Order of the reaction = \(\frac { 1 }{ 2 }\) + 2 = 2 \(\frac { 1 }{ 2 }\) or 0.5
Question 9.
The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will ¡t affect the rate of formation of Y?
Answer:
For the reaction, X → Y as it follows second order kinetics, therefore the rate law equation will be
Rate = k[X]2 = ka2
if [X] = a mol-1
if the concentration of X is increased three times, then
[X] = 3a mol L-1
∴ Rate = k (3a)2 = 9 ka2
Thus, the rate of the reaction will become 9 times. Hence the rate of formation of Y will increase by 9 times.
Question 10.
Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Answer:
For a first order reaction,

Question 11.
What will be the effect of temperature on rate constant?
Answer:
Rate constant of a reaction is nearly doubled with rise in temperature by 10°C. The exact dependence of the rate constant on temperature is given by Arrhenius equation:
Rate constant,
![]()
Question 12.
A reaction is first order in A and second order in B.
- Write the differential rate equation.
- How is the rate affected on increasing the concentration of B three times?
- How is the rate affected when the concentrations of both A and B arc doubled?
Answer:
- \(\frac { dx }{ dt }\) = k [A]1 [B]2
- If concentration of ‘B’ is tripled, then the rate will become 9 times.
- When concentration of both A and B are doubled, then the rate will become 8 times.
Question 13.
Define zero order reaction. Give the unit for its rate constant(k).
Answer:
Zero Order Reaction. The reaction in which the rate of reaction is independent of the concentration of the reactants is called zero order reaction.
Rate = k [A]0 ⇒ k
Where k is the rate constant. Its unit is mol L-1 s-1
Question 14.
Write units of rate constant k for zero order, first order, second order and n order reaction.
Answer:
Order of Reaction
- Zero order reaction
- First order reaction
- Second order reaction
- nth order reaction
Unit of k:
- mol L-1 s-1
- s-1
- mol L s-1
- (mol/ L)1-n s-1
Question 15.
What is the effect of catalyst on the activation energy? Why?
Answer:
A Catalyst lower down the activation energy. It provides an alternate path to the reaction. It forms an unstable intermediate which readily changes into products.
Question 16.
Give two differences between zero order and first order reaction.
Answer:
Zero Order:
- Its ‘k’ has unit = mol L-1 s-1
- Its t 1/2 is directly proportional to initial conc. of reactant
First order:
- Its ‘k’ has unit = time-1 = sec-1
- Its half life is independent of the initial conc. of the reactant.
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements 3 Mark Questions and Answers
Question 1.
Write the differences between the rate and rate constant of the reaction.
Answer:
Rate of a reaction:
- It represents the speed at which the reactants are converted into products at any instant
- It is measured as decrease in the concentration of the reactants (or) increase in the concentration of products
- It depends on the initial concentration of reactants
Rate constant of a reaction:
- It is a proportionality constant
- It is equal to the rate of the reaction, when the concentration of each of the reactants is unity
- It does not depend on the initial concentration of the reactants
Question 2.
What are the examples of first order reaction?
Answer:
- Decompostion of dinitrogen pentoxide
N2O2(g) → 2NO2(g) + \(\frac { 1 }{ 2 }\) O2(g) - Decomposition of thionylchloride
SO2Cl2(g) → SO2(g) + CI2(g) - Decomposition of H2O2 in aqueous solution
H2O2(aq) → H2O(1) + \(\frac { 1 }{ 2 }\) O2(g) - Isomerisation of cyclopropane to propene
Question 3.
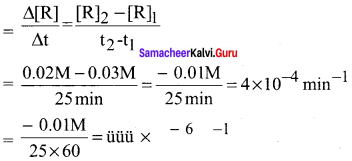
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Answer:
Question 4.
In a reaction, 2 A → products. the concentration of A decreases from 0.5 moI L-1 to 0.4 mol L-1 in 10 minutes. Calculate the rate during this interval.
Answer:
Average rate

Question 5.
A first order reaction has a rate constant, 1.15 x 10-3 s-1. How long will 5 g of this reactant take to reduce to 3 g?
Answer:
Here,
[R]0 = 5g
[R] = 3 g
k = 1.15 x 10-3 s-1 As the reaction is of first order,

Question 6.
Time required to decompose SO2CI2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction. For a first order reaction,
Answer:

Question 7.
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is-
- doubled
- reduced to half.
Answer:
1. Reaction is second order with respect to the reactant
∴ Rate = k[A]2 = ka2.
when [A] = 2a
Rate k (2a)2
= 4ka2
= 4 times
Therefore, when concentration of the reactant is doubled the rate will become 4 times
2. when [A] = \(\frac { 1 }{ 2 }\) a
Rate = k \({ \left( \frac { 1 }{ 2 } a \right) }^{ 2 }\) = \(\frac { 1 }{ 4 }\) ka2 = \(\frac { 1 }{ 4 }\) k
Therefore, rate will be reduced to one-fourth of the initial rate.
Question 8.
The rate constant for a first order reaction is 60 s1. How much time will It take to reduce the initial concentration of the reactant to its 1/6th value?
Answer:
![]()
Question 9.
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
Answer:
For a first order reaction, t = k = \(\frac { 2.303 }{ t }\) log \(\frac { a }{ a-x }\)
99% completion means that
x = 99% of a =0.99 a
t99% = \(\frac { 2303 }{ k }\) log \(\frac { a }{ a-0.99a }\) = \(\frac { 2.303 }{ k }\) log 102 = 2 x \(\frac { 2303 }{ k }\)
90% completion means that
x = 90% of a = 0.90 a
t99% = \(\frac { 2303 }{ k }\) log \(\frac { a }{ a-0.99a }\) = \(\frac { 2.303 }{ k }\) log 10 = \(\frac { 2.303 }{ k }\)
\(\frac{t_{99 \%}}{t_{90 \%}}=\left(\frac{2 \times 2303}{k}\right) / \frac{2.303}{k}=2\)
or
t99% = 2 x t90%
Question 10.
Calculate the half life of a first order reaction whose rate constant is 200 s-1
Answer:
Here rate constant
k= 200 s-1
∴ Half – life of a first order reaction is
t1/2 = \(\frac { 0.693 }{ k }\) = \(\frac { 0.693 }{ 200 }\) = 3.46 x 10-3 sec
Question 11.
The decomposition of dinitrogen pentoxide (N2O) follows the first order rate law. Calculate the rate constant from the given data.
t = 800 sec, [N2O5] = 1.45 moI L-1 = [A2]
t = 1600 sec
[N2O3] = 0.88 moI L-1 = [A2]
Answer:
Applying the formula,
k = \(\frac { 2.303 }{ \left( { t }_{ 2 }-{ t }_{ 1 } \right) }\) log 10 \(\frac { \left[ { A }_{ 1 } \right] }{ \left[ { A }_{ 2 } \right] }\)
= \(\frac { 2.303 }{ (1600 – 800) }\) log 10 \(\frac { 1.45 }{ 0.88 }\) = \(\frac { 2.303 }{ 800 }\) x 0.2169
= 6.24 x 10-4 sec-1
Question 12.
A second order reaction in which both the reactants have same concentration, is 20% completed in 500 seconds. How much time it will take for 60% completion?
Answer:
The second order equation when both the reactants have same concentration is
k = \(\frac { 1 }{ t }\). \(\frac { x }{ a(a – x) }\)
If a = 100, x = 20, 1= 500 seconds
Then k = \(\frac { 1 }{ 500 }\) x \(\frac { 20 }{ 100 x (100-20) }\)
When
a = 100
x = 60
t = ?
t = \(\frac { 1 }{ k }\) \(\frac { 60 }{ 100 x 40 }\)
Substituting the value of k,
t = \(\frac { 500 x 100 x 80 }{ 20 }\) x \(\frac { 60 }{ 100 x 40 }\)
or
t = 3000 seconds
Question 13.
A first order reaction is 20% completed in 10 minutes. Calculate the time taken for the reaction to go to 80% completion.
Answer:
Applying the first order equation,
k = \(\frac { 2303 }{ t }\) l0g \(\frac { { \left[ R \right] }_{ 0 } }{ \left[ R \right] }\)
At t = 10 min
R = 100 – 20
k = \(\frac { 2303 }{ t }\) log 10 \(\frac { 100 }{ (100 – 20) }\)
t = \(\frac { 2303 }{ 10 }\) log 10 \(\frac { 100 }{ 80 }\)
= 0.0223 min-1
Question 14.
For a reaction: 2NH3(g) \(\underrightarrow { Pt }\) N2(g)+ 3H2(g) Rate = K
- Write the order and molecularity of this reaction.
- Write the unit of K.
Answer:
- Order of reaction Zero order. Molecularity = 2
- Unit of K = mol L-1 sec-1
Samacheer Kalvi 12th Chemistry Chemical Kinetics Elements 5 Marks Questions and Answers
Question 1.
How would you calculate the order of the reaction 2NO + O2(g) → 2NO2(g) by an experiment?
(or)
prove that 2NO + O2 → 2 NO2 is a third order reaction.
Answer:
2NO(g) + O2(g) → 2NO2(g)
Series of experiments are conducted by keeping the concentration of one of the reactants as constant and changing the concentration of the others.

Rate = k [NO]m[O2]n
For experiment 1, the rate law is
Rate1 = k [NO]m [O2]n
19.26 x 10-2 = k [1.3]m [1.1]n ………………….(1)
For experiment 2
Rate2 = k [NO]m [O2]n
38.40 x 10-2 = k [1.3]m [2.2]n ……………….(2)
For experiment 3
Rate3 = k [NO]m [O2]n
76.8 x 10-2 = k [2.6]m [1.1]n ………………(3)
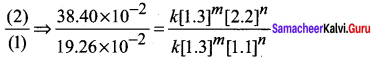
2 = \({ \left( \frac { 2.2 }{ 1.1 } \right) }^{ n }\)
4 = 2m
⇒ n = 1
Therefore the reaction is first order with respect to O2
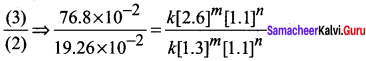
2 = \({ \left( \frac { 2.6 }{ 1.3 } \right) }^{ m }\)
4 = 2m
⇒ m = 1
Therefore the reaction is second order with respect to NO
The rate law is Rate– = k [NO]2 [O2]1
The overall order of the reaction = 2 + 1 = 3
Question 2.
Derive the integrated rate law for a first order reaction?
Answer:
A reaction whose rate depends on the reactant concentration raised to the first power is called a first order reaction. First order reaction is A → product. Rate law can be expressed as, Rate = k [A]1. Where, k is the first order rate constant
\(\frac { -d[A] }{ dt }\) = k[A]1
\(\frac { -d[A] }{ [A] }\) = k.dt ……………………(1)
Integrate the above equation (I) between the limits of time t = 0 and time equal to t, while the concentration varies from initial concentration [A0] to [A] at the later time.
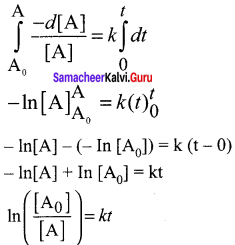
This equation (2) is in natural logarithm. To convert it into usual logarithm with base 10, we have to multiply the term by 2.303
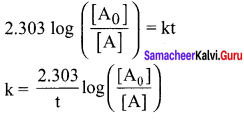
Question 3.
Explain the effect of temperature on reaction rate based on Arrhenius theory.
Answer:
1. Generally, the rate of a reaction increases with increasing temperature. However, there are very few exceptions.
2. As a rough rule, for many reactions near room temperature, reaction rate tends to double when the temperature is increased by 10°C.
3. A large number of reactions are known which do not take place at room temperature but occur readily at higher temperature. Example – Reaction between H2 and O2 to form H2O takes place only when an electric spark is passed.
4. Arrhenius suggested that the rates of most reactions vary with temperature in such a way that the rate constant is directly proportional to
![]() and he proposed a relation between the rate constant and temperature.
and he proposed a relation between the rate constant and temperature.
![]()
where
k = frequency factor
R = gas constant ,
Ea = Activation energy
T = Absolute temperature (in kelvin)
The factor A does not vary significantly with temperature and hence it may be taken as a constant.
5. Taking logarithm on both side of the equation (1)

6. The plot of Ink vs \((\frac { 1 }{ T })\) is a straight line with negative slope\(\frac { { E }_{ a } }{ RT }\). If the rate constant for a reaction at two different temperatures is known, we can calculate the activation energy.
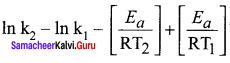
This equation can be used to calculate E from rate Ea constants k1 & k2 at temperature T1 and T2
Question 4.
Explain about the factors that affecting the reaction rate.
Answer:
The rate of a reaction is affected by the following factors.
1. Nature and state of the reactant
(a) A chemical reaction involves breaking of certain existing bonds of the reactant and forming new bonds which lead to the product. The net energy involved in this process is dependent on the nature of the reactant and hence the rates are different for different reactants.
(b) Gas phase reactions are faster as compared to the reactions involving solid or liquid reactants. For example, reaction of sodium metal with iodine vapours is faster than the reaction between solid sodium and solid iodine.
2. Concentration of the reactant
The rate of the reaction increases with the increase in the concentration of the reactants. According to collision theory, the rate of the reaction depends upon the number of collisions between the reacting molecules. Higher the concentration, greater is the possibility for collision and hence the rate.
3. Effect of surface area of the reactant:
In heterogeneous reactions, the surface areas of the solid reactants plays an important role in deciding the rate. For a given mass of a reactant, when the particle size decreases surface area increases.
Increase in surface area of reactant leads to more collisions per litre per second and hence the rate of reaction is increased. For example, powdered calcium carbonate reacts much faster with dilute HCI than with the same mass of CaCOl as marble
4. Temperature:
For many reactions near room temperature, the reaction rate tends to double when the temperature is increased by 10°C . For eg, Reaction between H2 and O2 to form H2O take place only when an electric spark is passed. So when the temperature increases, the rate of the reaction also increases.
5. Effect of presence of catalyst
(a) A catalyst is substance which alters the rate of a reaction without itself undergoing any permanent chemical change. They may participate in the reaction, but again regenerated and the end of the reaction.
(b) In the presence of a catalyst, the energy of activation is lowered and hence greater number of molecules can cross the energy barrier and change over to products,thereby increasing the rate of the reaction.
Question 5.
The decomposition of A into product has value of k as 4.5 x 103 s-1 at 10°C and energy of activation 60 kJ mol-1. At what temperature would k be 1.5 x 104 s-1 ?
Answer:
k1 = 4.5 x 103 s-1
T1 = 10 + 273 K = 283 K
k2 = 1.5 x 104 s-1
T2 = ?
Ea = 6o kJ mol-1
According to Arrhenius equation
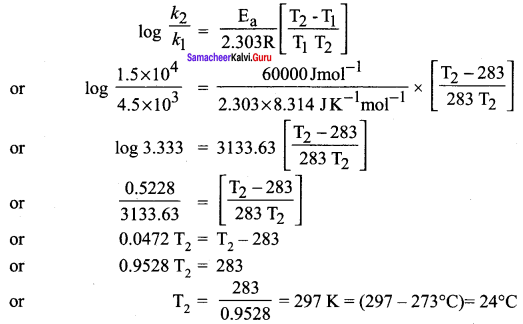
Question 6.
For a decomposition reaction the values of rate constant k at two different temperatures are given below:
k1 = 2.15 x 10 L mol-1 s-1 at 650 K
k2 = 2.39 x 10 L mol-1 s-1 at 700 K
Calculate the value of activation energy for this reaction. (R = 8.314 J K-1 mol-1 )
Answer:
Here
k1 = 2.15 x 10 L mol-1 s-1 at 650 K
T1 = 650 K
T2 = 700K and
k2 = 2.39 x 10 L mol-1 s-1 at 700 K
R = 8.314 J K-1 mol-1
Using the formula
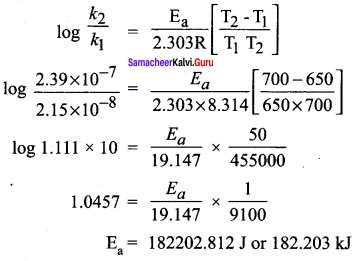
Question 7.
For a certain chemical reaction variation In concentration, In IRI Vs time (s) plot is given below.
For this reaction write/draw:
- What is the order of the reaction?
- What is the units of rate constant (k)?
- Give the relationship between k and t1/2 (half-life period).
- What does the slope of above line indicate?
- Draw the plot of log [R0]/[R] vs time (s)
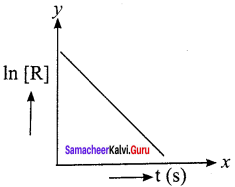
Answer:
1. First order
2. time-1 (s-1)
3. 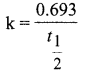
4. Rate constat (k) of reaction
5. 
Question 8.
A substance reacts according to the first order rate law and the specific reaction rate for the reaction ¡s 1 x 10-2 s-1. If the initial concentration is 1.0 M.
- What is the initial rate?
- What ¡s the reaction rate after 1 minute?
Answer:
1. Initial rate of a first order reaction
= k C
= l x 10-2 x 1.0
= l x 10-2 mol L-1 s-1
2. Concentration after 60 seconds is calculated by applying the first order kinetic equation,
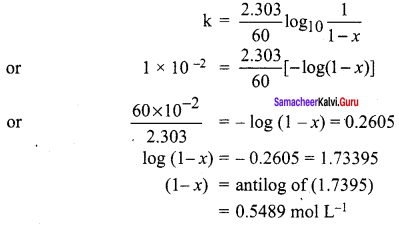
Rate of reaction after 1 minute
= k x C
= l x 10-2 x 0.5489
5.489 x 10-3 mol L-1 s-1
Question 9.
A first order reaction is 50% completed in 30 minutes at 27°C and in 10 minutes at 47°C. Calculate the reaction rate constant at 27°C and the energy of activation of the reaction in kJ mol-1
Answer:
For a first order reaction

Now applying the following equation:
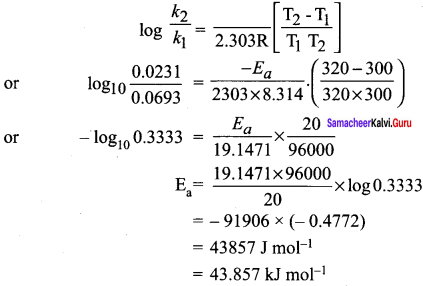
Question 10.
In Arrhenius equation for a certain reaction, the values of A and E (activation energy) are 4 x 1013 sec-1 and 98.6 KJ mol-1 respectively. If the reaction is of first order, at what temperature will its half life period be 10 minutes?
Answer:
According to the Arrhenius equation.

For a first order reaction
t1/2 = \(\frac { 0.693 }{ k }\) \(\frac { 0.693 }{ 600 }\)
So,
k = \(\frac { 0.693 }{ 600 }\) sec-1 (t1/2 = 10 min = 600 sec)
= 1.1 x 10-3 sec-1
Hence, log (1.1 x 10-3)
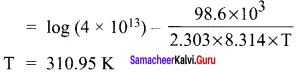
Common Errors
Common Errors:
- Order and molecularity may get confused
- Unit of first order Rate constant and zero order may get con fused.
- t1/2 – Half liefe period may be difficult to remember
Rectifications:
- Order and molecularity are same for the single step process. But for reactions of more than one step, they may be different.
- First order sec-1, Zero order – mol litre-1 sec-1
- t1/2 = 0.693 / k1 for first order reaction.
We hope the Class 12th Samacheer Kalvi Chemistry Solutions Chapter 7 Chemical Kinetics Book Solutions Answers Guide Solutions provided here help the students to get the best knowledge. Also, students can use our Samacheer Kalvi Class 12th Chemistry Solutions Chapter 7 Chemical Kinetics Questions and Answers for better practice. You can contact us at any moment by giving comments in the comment section. Keep checking updates by bookmarking our website.