Tamilnadu State Board Solutions for Class 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Questions and Answers will help you to improve the complete subject knowledge. Every student must look at the single concept included in Samacheer Kalvi 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions Answers Guide Solutions PDF. All the concepts are explained clearly with examples and pictures.
Students can easily avoid the struggle to get the best book for Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions Answers Guide learning. You have to go through Chapterwise Samacheer Kalvi Class 12th Textbook Solutions for Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions for better practice.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds
Students who wish to have the strong basics of Chemistry Solutions Chapter 13 Organic Nitrogen Compounds can use Tamilnadu State Board Class 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Questions and Answers Guide pdf. Enhance your knowledge by referring to the Samacheer Kalvi Solutions pdf. We provided a free pdf of Tamilnadu State Board Class 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions Answers Guide material and textbook for students. Check it out now and start preparing for the exam immediately. Score maximum marks in the exam by referring to Samacheer Kalvi Class 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions Answers Guide Solutions Pdf.
Samacheer Kalvi 12th Chemistry Chapter 13 Organic Nitrogen Compounds Text Book Evaluation
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds Multiple Choice Questions
Question 1.
Which of the following reagent can be used to convert nitrobenzene to aniline?
(a) Sn / HCl
(b) ZnHg / NaOH
(c) LiAIH4
(d) All of these
Answer:
(a) Sn / HCl
Question 2.
The method by which aniline cannot be prepared is ……………
(a) degradation of benzamide with Br2 / NaOH
(b) potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution.
(c) Hydrolysis of phenylcyanide with acidic solution
(d) reduction of nitrobenzene by Sn / HCI
Answer:
(b) potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution.
Question 3.
Which one of the following will not undergo Hofmann bromamide reaction?
(a) CH3CONHCH3
(b) CH3CH2CONH2
(c) CH3CONH2
(d) C6H5CONH2
Answer:
(a) CH3CONHCH3
Only primary amides undergo hoffmann bromamide reaction
Question 4.
Assertion : Acetamide on reaction with KOH and bromine gives acetic acid
Reason : Bromine catalyses hydrolysis of acetamide.
(a) if both assertion and reason are true and reason is the correct explanation of assertion,
(b) if both assertion and reason are true but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(d) both assertion and reason are false
Question 5.
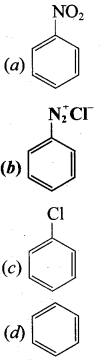
![]()
(a) bromomethane
(b) a – bromo sodium acetate
(c) methanamine
(d) acetamide
Answer:
(c) methanamine
![]()
Question 6.
Which one of the following nitro compounds does not react with nitrous acid?
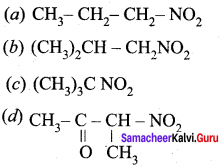
Answer:
(c) (CH3)3CNO2 – 30 nitroalkane
Question 7.
![]() this reaction is known as ………………
this reaction is known as ………………
(a) Friedel – crafts reaction
(b) HVZ reaction
(c) Schotten – Baumann reaction
(d) none of these
Answer:
(c) Schotten – Baumann reaction
Question 8.
The product formed by the reaction an aldehyde with a primary amine
(a) carboxylic acid
(b) aromatic acid
(c) schiff ‘s base
(d) ketone
Answer:
(c) schiff ‘s base
Question 9.
Which of the following reaction is not correct.
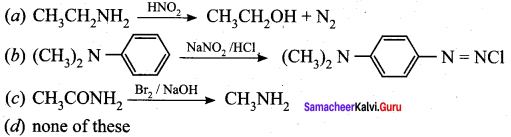
Answer:
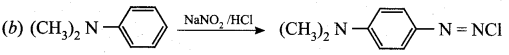
P – nitrosation takes places, the product is ![]()
Question 10.
When aniline reacts with acetic anhydride the product formed is …………….
(a) o – aminoacetophenone
(b) m – aminoacetophcnone
(c) p – aminoacetophenone
(d) acetanilide
Answer:
(d) acetanilide
Question 11.
The order of basic strength for methyl substituted amine solution is ………….
(a) N(CH3)3 > N(CH3)2H > N(CH3)H2 > NH3
(b) N(CH3)H2 > N(CH3)2H > N(CH3)3 > NH3
(c) NH3 > N(CH3)H2 > N(CH3)2H > N(CH3)3
(d) N(CH3)2H > N(CH3)H2 > N(CH3)3 > NH3
Answer:
(d) N(CH3)2H > N(CH3)H2 > N(CH3)3 > NH3
Question 12.
(a) H3PO2 and H2O
(b) H+ / H2O
(c) HgSO4 / H2SO4
(d) Cu2Cl2
Answer:
(a) H3PO2 and H2O
Question 13.
![]()
(a) C6H5 – OH
(b) C6H5 – CH2OH
(c) C6 H5 – CHO
(d) C6H5NH2
Answer:
(a) C6H5 – OH
![]()
Question 14.
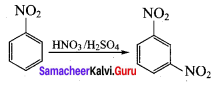
Nitrobenzene on reaction with at 80 – 100°C forms which one of the following products?
(a) 1, 4 – dinitrobenzene
(b) 2, 4, 6 – tirnitrobenzene
(c) 1, 2 – dinitrobenzene
(d) 1, 3 – dinitrobenzene
Answer:
(d) 1, 3 – dinitrobenzene
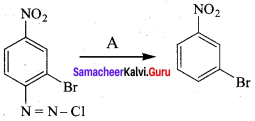
Question 15.
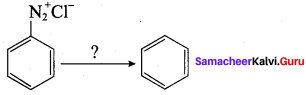
C5H13N reacts with HNO2 to give an optically active compound – The compound is …………..
(a) pentan – 1 – amine
(b) pentan – 2 – amine
(c) N,N – dimethylpropan – 2 – amine
(d) N – methylbutan – 2 – amine
Answer:
(d) N – methylbutan – 2 – amine

Question 16.
Secondary nitro alkanes react with nitrous acid to form …………..
(a) red solution
(b) blue solution
(c) green solution
(d) yellow solution
Answer:
(b) blue solution
Question 17.
Which of the following amines does not undergo acetylation?
(a) t – butylamine
(b) ethylamine
(c) diethylamine
(d) triethylamine
Answer:
(d) triethyl amine (3°amine)
Question 18.
Which one of the following is most basic?
(a) 2, 4 – dichloroaniline
(b) 2, 4 – dimethyl aniline
(c) 2, 4 – dinitroaniline
(d) 2, 4 – dibromoaniline
Answer:
(b) 2, 4 – dimethyl aniline
CH3 is a +1 group, all other – I group. +1 group increase the electron density on NH2 and hence increases the basic nature.
Question 19.
When
![]()
is reduced with Sn / HCI the pair of compounds formed are ………..
(a) Ethanol, hydrozylamme hydrochloride
(b) Ethanol, ammonium hydroxide
(c) Ethanol, NH2OH
(d) C3H5NH2, H2O
Answer:
(a) Ethanol, hydrozylamine hydrochloride
Question 20.
TUPAC name for the amine
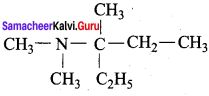 is ………………
is ………………
(a) 3 – Bimethylamino – 3 – methyl pentane
(b) 3 (N,N – Triethyl) – 3 – amino pentane
(c) 3 – N, N – trimethyl pentanamine
(d) 3 – (N, N – Dimethyl amino) – 3 – methyl pentane
Answer:
(d) 3 – (N, N – Dimethyl amino) – 3 – methyl pentane
Question 21.
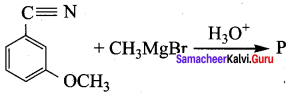
Product ‘P’ in the above reaction is ………………
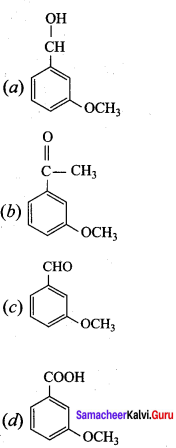
Answer:
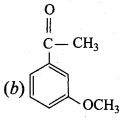
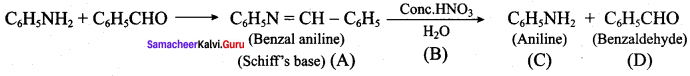
Question 22.
Ammonium salt of bcnzoic acid is heated strongly and the product so formed is reduced and then treated with NaNO2 / HCl at low temperature. The final compound formed is ……………
(a) Benzene diazonium chloride
(b) Benzyl alcohol
(c) Phenol
(d) Nitrosobenzene
Answer:
(b) Benzyl alcohol
![]()
Question 23.
Identify X in the sequence give below.
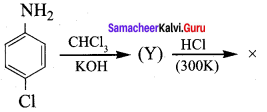 + Methanoic acid
+ Methanoic acid
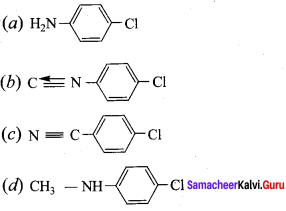
Answer:
![]()
Question 24.
Among the following, the reaction that proceeds through an electrophilic substitution, is ……………..
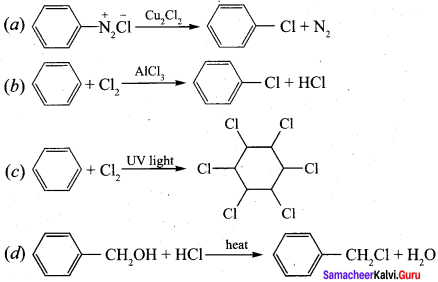
Answer:
Question 25.
The major product of the following reaction
Answer:
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds Short Answer Questions
Question 1.
Write down the possible isomers of lthe C4H9NO2 give their IUPA names.
Answer:

Question 2.
There are two isomers with the formula CH3NO2. How will you distinguish between them?
Answer:
CH3NO2 has two isomers. They are
1. CH3 – NO2 (Nitromethane)
2.
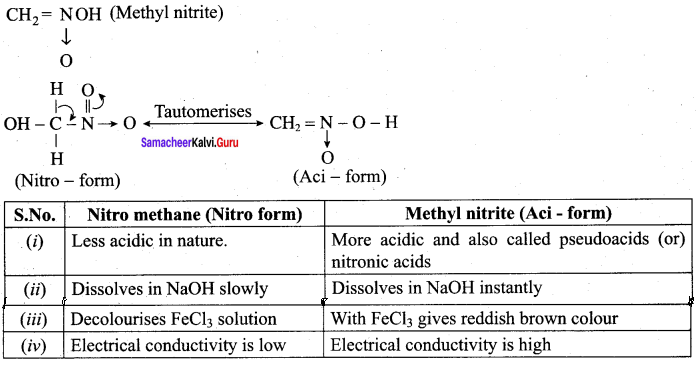
Question 3.
What happens when
- 2 – Nitropropane boiled with HCI
- Nitrobenezen electrolytic reduction in strongly acidic medium.
- Oxidation of tert – butylamine with KMnO4
- Oxidation of acetoneoxime with triuluoroperoxy acetic acid.
Answer:
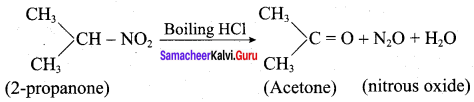
1. 2 – Nitropropane boiled with HCI:
2 – nitropropane upon hydrolysis with boiling HCl give a ketone (2 – propanone) and nitrous oxide.
2. Nitrobenezen electrolytic reduction in strongly acidic medium:
Electrolytic reduction of nitrobenzene in weakly acidic medium gives aniline but in strongly acidic medium, it gives p – aminophenol obviously through the acid – cataLysed rearrangement of the initially formed phenyihydroxylamine.
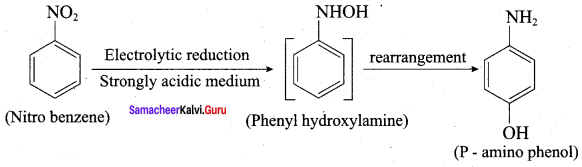
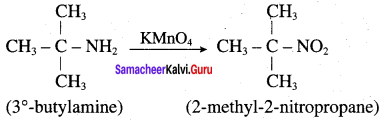
3. Oxidation of tert – butylamine with XMnO4:
In general, primary amines, in which the – NH2 group is attached to a tertiary carbon can be oxidised with KMnO4 to the corresponding nitro compound in excellent yield. Therefore 3° – butylamine oxidised to give 2 – methyl – 2 – nitropropane.
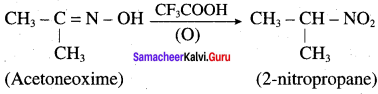
4. Oxidation ofacetoneoxime with trifluoroperoxy acetic acid:
Oxidation ofacetoneoxime with trifluoroperoxy acetic acid gives 2 – nitropropane.
Question 4.
How will you convert nitrobenzene into
- 1, 3, 5 – trinitrobenzene
- o and p – nitrophenol
- m – nitro aniline
- azoxybenzene
- hydrozabenzene
- N – phenylhydroxylamine
- aniline
Answer:
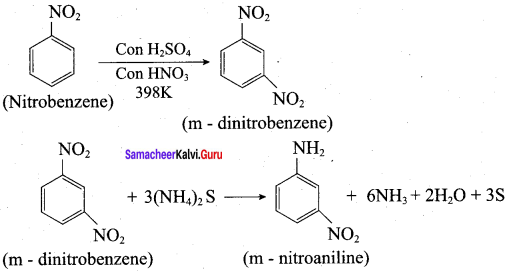
1. Conversion of nitrobenzene into 1, 3, 5 – trinitrobenzene:
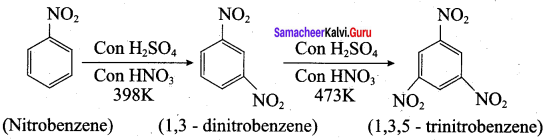
2. Conversion of nitrobenzene into o and p – nitrophenol:
(a) Method I:
Nitrobenzene heated with solid KOH at 340 K gives a low yield of a mixture of 0 – and P – nitrophenols.
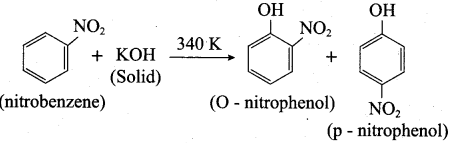
(b) Method II:
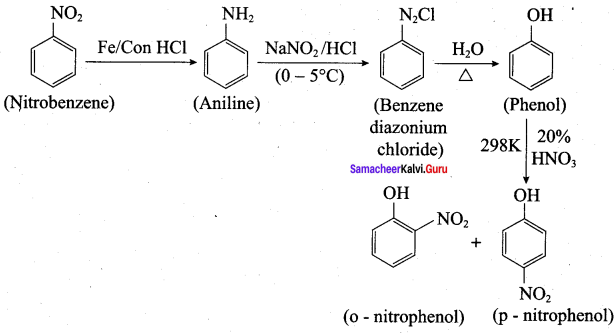
3. Conversion of nitrobenzene into m – nitro aniline:
4. Conversion of nitrobenzene into azoxybenzene:
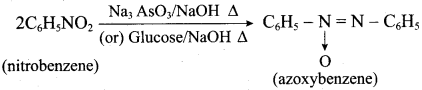
5. Conversion of nitrobenzene into hydrazobenzene:
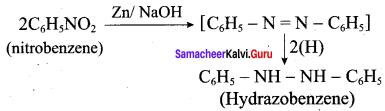
6. Conversion of nitrobenzene into N – phenylhydrozylamine:
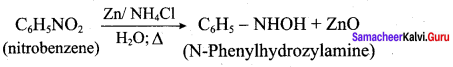
7. Conversin of nitrobenzene into aniline:
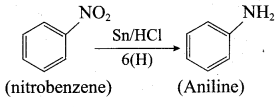
Question 5.
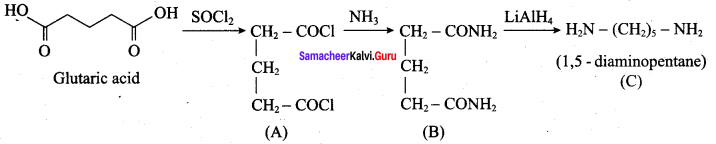
Identify compounds A,B and C in the following sequence of reactions.
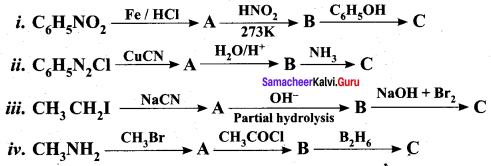
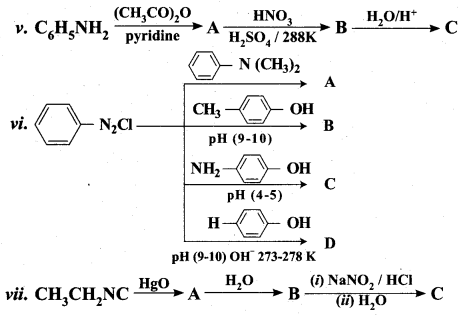
Answer:
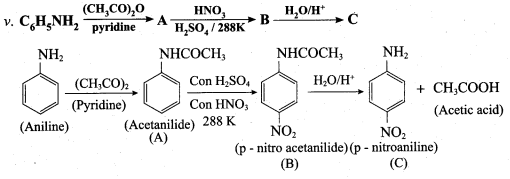
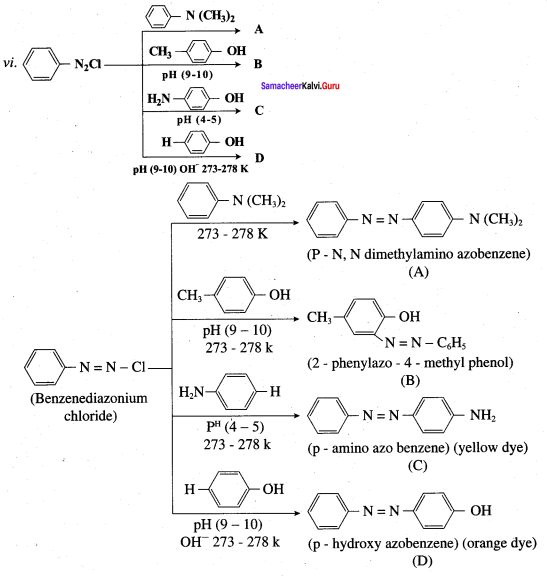
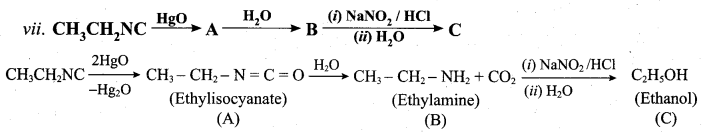
Question 6.
Write short flotes on the following
- Hoffmann’s bromide reaction
- Ammonolysis
- Gabriel phthalimide synthesis
- Schotten – Baumann reaction
- Carbylamine reaction
- Mustard oil reaction
- Coupling reaction
- Diazotisation
- Gomberg reaction
Answer:
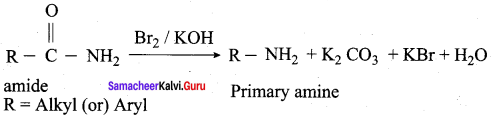
1. Hoffmann’s bromide reaction:
When Amides are treated with bromine in the presence of aqueous or ethanolic solution of KOH, primary amines with one carbon atom less than the parent amides are obtained.
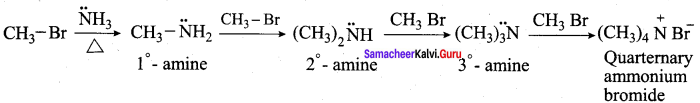
2. Ammonolysis:
When Alkyl halides (or) benzylhalides are heated with alcoholic ammonia in a sealed tube, mixtures of 1°, 2° and 3° amines and quaternary ammonium salts are obtained.
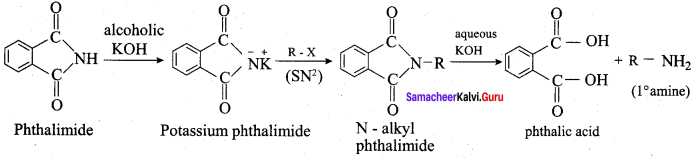
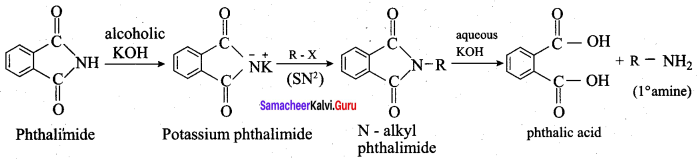
3. Gabriel phthalimide synthesis:
Gabriel synthesis is used for the preparation of Aliphatic primary amines. Phthalimide on treatment with ethanolic KOH forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis gives primary amine.
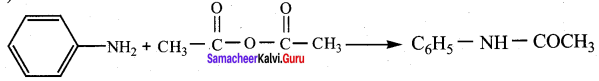
4. Schotten – Baumann reaction:
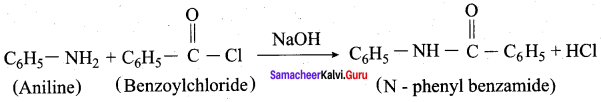
Aniline reacts with benzoylchloride (C6H5COCl) in the presence of NaOH to give N – phenyl benzamide. This reaction is known as Schotten Baumann reaction. The acylation and benzoylation are nucleophilic substitutions.
5. Carbylamine reaction:
Aliphatic (or) aromatic primary amines react with chloroform and alcoholic KOH to give isocyanides (carbylamines), which has an unpleasant smell. This reaction is known as carbylamines test. This test used to identify the primary amines.
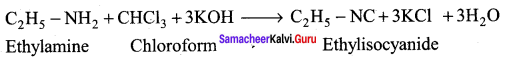
5. Mustard oil reaction:
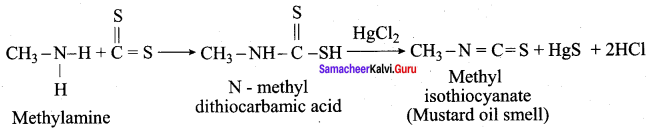
When primary amines are treated with carbon disuiphide (CS2), N – alkyldithio carbonic acid is formed which on subsequent treatment with HgCI2, give an alkyl isothiocyanate.
6. Coupling reaction:
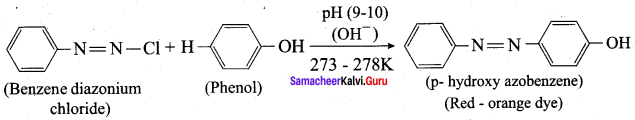
Benzene diazonium chloride reacts with electron-rich aromatic compounds like phenol, aniline to form brightly coloured azo compounds. Coupling generally occurs at the para position. If para position is occupied then coupling occurs at the ortho position. Coupling tendency is enhanced if an electron-donating group is present at the para – position to ![]() group. This is an electrophilic substitution.
group. This is an electrophilic substitution.
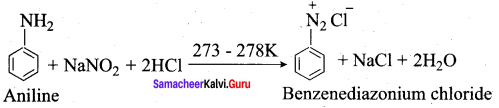
7. Diazotisation:
Aniline reacts with nitrous acid at low temperature (273 – 278 K) to give benzene diazonium chloride which is stable for a short time and slowly decompose seven at low temperatures. This reaction is known as diazotization.
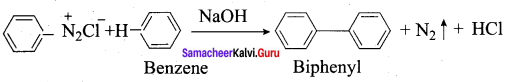
8. Gomberg reaction
Benzene diazonium chloride reacts with benzene in the presence of sodium hydroxide to give biphenyl. This reaction in known as the Gomberg reaction.
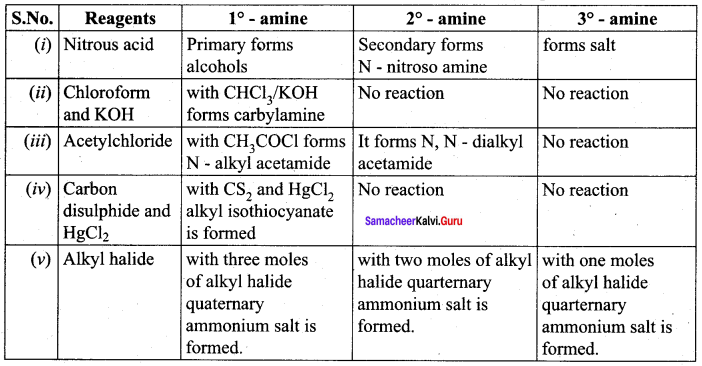
Question 7.
How will you distinguish between primary secondary and tertiary alphatic amines.
Answer:
Question 8.
Account for the following
- Aniline does not undergo Friedel – Crafts reaction
- Diazonium salts of aromatic amines are more stable than those of aliphatic amines
- pkb of aniline is more than that of methy lamine
- Gabriel phthalimide synthesis is preferred for synthesising primary amines.
- Ethylamine is soluble in water whereas aniline is not
- Amines are more basic than amides
- Although amino group is o – and p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m – nitroaniline.
Answer:
1. Aniline does not undergo Friedel – Crafts reaction:
Aniline being a Lewis base reacts with Lewis acid AiCl3 to form a salt.
![]()
Due to the presence of a positive charge on N – atom in the salt the group ![]() acts as a strongly deactivating group. As a result, it reduces the electron density in the benzene ring and which inhibits the electrophilic substitution reaction. Therefore aniline does not under go Friedel – Crafts reaction.
acts as a strongly deactivating group. As a result, it reduces the electron density in the benzene ring and which inhibits the electrophilic substitution reaction. Therefore aniline does not under go Friedel – Crafts reaction.
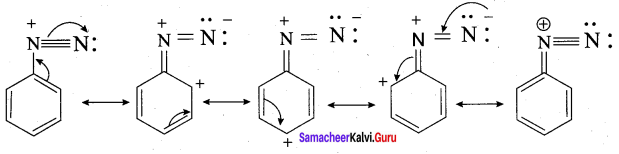
2. Diazonium salts of aromatic amines are more stable than those of aliphatic amines:
The diazonium salts of aromatic amines are more stable than those of aliphatic amines due to dispersal of the positive charge on the benzene ring as shown below.
3. pKb of aniline is more than that of methylamine:
In aniline, the lone pair of electrons on the N – atom is delocalized over the benzene ring. As a result electron density on the nitrogen decreases. In contrast in CH3NH2, +I effect of CH3 increases the electron density on the N-atom. Therefore, aniline is a weaker base than methylamine and hence its pK value is more than that of methyl amine.
4. Gabriel phthalimide synthesis is preferred for synthesising primary amines:
Gabriel phthalimide reaction gives pure 10 amine without any contamination of 2° and 3°-amines. Therefore it is preferred for synthesising primary amines.
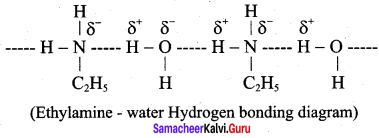
5. Ethylamine is soluble in water whereas aniline is not:
Ethylamine when added to water forms intermolecular H – bonds with water. And therefore it is soluble in water. But aniline does not form H – bond with water to a very large extent due to the presence of a large hydrophobic – C6H5 group. Hence, aniline is insoluble in water.
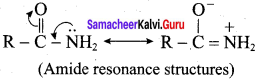
6. Amines are more basic than amides:
In simple amines, the lone pair of electrons is on nitrogen and hence available for protonation. In amides on the other hand, the electron pair on nitrogen is delocalised to the carboxyl oxygen through resonance and thus it is not available for protonation. So amines are more basic than amides.
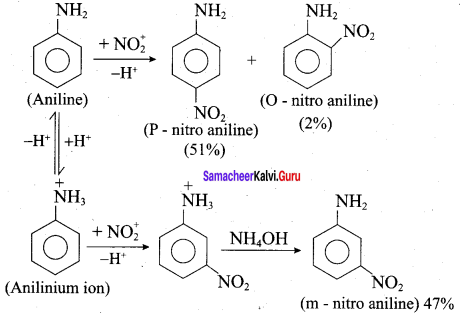
7. Although amino group is o – and p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m – nitroaniline:
Nitration is usually carried out with a mixture of conc HNO3 and conc H2SO4. In the presence of these acids, most of aniline gets protonated to form anilinium ion. Therefore, in the presence of acids, the reaction mixture consists of aniline and anilinium ion.
Now – NH2, group in aniline is O, P – directing and activating while the – NH3 group is anilinium ion is meta – directing and deactivating. Whereas nitration of aniline (due to steric hindrance at o – position) mainly gives p-nitroaniline, the nitration of anilinium ion gives m – nitro aniline. In actual practice, approximately a 1 : 1 mixture of P and m – nitroaniline is formed.
Question 9.
Arrange the following
- In increasing order of solubility in water, C6H5NH2, (C2H5)2NH , C2H5NH2
- In increasing order of basic strength
- aniline, p – toludine and p – nitroaniline
- C6H5NH2, C6H5NHCH3, C6H5NH2, p – Cl – C6H4 – NH2
- In decreasing order of basic strength in gas phase.
C2H5NH2, (C2H5)3NH , (C2H5)3N and NH3 - In Increasing order of boiling point
C2H5OH , (CH3)2NH, C2H5NH2 - In decreasing order of the pKb values
C2H5NH2, C6H5NHCH3, (C2H)2NH4 and CH3NH2 - Increasing order of basic strength C6H5NH2, C6H5N(CH3)2, (C6H5)2NH and CH3NH2
- In decreasing order of basic strength
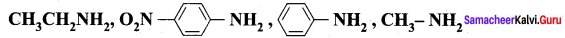
Answer:
1. Solubility decreases with increase in molecular mass of amines due to increase in the size of a hydrophobic hydrocarbon part and with decrease in the number of H – atoms on the N – atom which undergo H – bonding.
Now among the given compounds C6H5NH2 has the highest molecular mass of 93 followed by (C2H5)2NH with molecular mass of 73 with C2H5NH2 has the lowest molecular mass of 45. Thus the solubility increases in the order in which molecular mass decreases.
2. (a) The electron – donating groups increases the basic strength of amines while the electron – withdrawing groups decrease the basic strength of amines. Therefore p – nitroaniline is the weakest base followed by aniline while p – toluidine, which has methyl group and therefore it is the strongest base. Basic strength increases in the order. P – nitro aniline < aniline < p – toluidine
(b) Chlorine atom has both – I effect and + R effect since – I effect out weights the + R effect, therefore p – chloro aniline is weak base than aniline. Aikyl groups are electron – donating groups. ”
As a result the electron density on the nitrogen atom increases in the ethylamine and thus they can donate lone pair of electrons niore easily. Therefore Ethylamine is more base than aromatic amines.
Due to delocalization of lone pair of electrons of the N – atom over the benzene ring, C6H5NH, and C6H5NHCH3 are far less basic than C2H5NH2. Further due to +1 effect of the CH3 group, C6H5NHCH3 is little more basic than C6H5NH2. Therefore increasing order basic strength is
3. In the gas phase, solvent effects i.e., stabilization of the conjugate acids due to H – bonding, are absent. Therefore, in the gas phase, basic strength mainly depends upon the +1 effect of the alkyl groups. Since the +1 effect increases with the number of ailcyl groups,
therefore the basic strength of the amines decreases as the number of ethyl groups decreases from three in (C2H5)3N to two in (C2H5)2NH to one in C2H5NH2 and zero in NH3. Basic strength in the gas phase decreases in the order is,
(C2H5)3N > (C2H5)2N > C2H5NH2 > NH3
4. Since the electro negativity of O is higher than that of N, therefore, alcohols form stronger H – 0bonds than amines. In other words, the boiling points of alcohols are higher than those of amines of comparable molecular masses. Therefore the boiling point of C2H5OH (46) is higher than those of (CH3)2NH (45) and C2H5NH2 (45).
Further since the extent of H – bonding depends upon the number of H-atoms on the N-atom. Therefore 1° – amines with two H – atoms on the N – atom have higher boiling points than 2° – amines having only one H – atom. Therefore the boiling point of C2H5NH2 is higher than that of(CH3)2NH. Increasing order of boiling point is,
(CH3)2NH < C2H5NH < CH5OH
5. Due to delocalization of lone pair of electrons of the N – atom over the benzene ring, C6H5NHCH3 is far less basic than C2H5NH2, (C6H5)2NH and CH3NH2. Among C2H5NH2 and (C2H5), NH, (C2H5)2NH is more basic than C2H5NH2 due to greater +1 effect of the two C2H5 groups and stabilization of its conjugate acid by H – bonding.
Compare to Ethyl and methyl group, C2H5 – group has more +1 effect than CH3 – group. Therefore methylamine is weak base than ethylamine. Combining all these facts the relative basic strength of these four amines decreases in the order.
(C2H5)2NH > C2H5NH2 > CH3NH2 > C6H5NHCH3. Since a stronger base has a lower pKb value therefore, pKb values decrease in the reverse order. C6H5NHCH3 > CH3NH2 > C2H5NH2 > (C2H5)2NH
6. Due to delocalization of lone pair of electrons of the N-atom over the benzene ring, all aromatic amines are less basic than alkylamines i.e., CH3NH2. Presence of electron – donating groups ( – CH3) on the N – atom increases the basicity of substituted aniline with respect to C6H5NH2.
In (C6H5)2NH, the lone pair of electrons on the N – atom is delocalized over two benzene rings instead of one in C6H5NH2, therefore (C6H5)2NH is much less basic than C6H5NH2. Combining all the three trends together, the basic strength of the four amines increasing in the order.
(C6H5)2NH < C6H5NH2 < C6H5N (CH3)2 < CH3NH,
7. Aliphatic amines are more basic than aromatic amines. Therefore CH3CH2NH2 and CH3NH2 are more basic. Among the ethylamine and methylamine. ethylamine was experienced more +1 effect than methylamine and hence ethylamine is more basic than methylamine.
Nitrogroup has a powerful electron withdrawing group and they have both – R effect as well as – I effect. As a result, all the nitro anilines are weaker bases than aniline. In P – nitroaniline
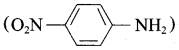
both – R effect and – I effect of the NO2 group decrease the basicity. Therefore decreasing order of basic strength is,
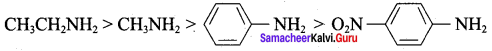
Ethylamine > Methylamine > Aniline > p – nitro aniline
Question 10.
How will you prepare propan – 1 – amine from
- butane nitrile
- propanamide
- 1 – nitropropane
Answer:
1. Preparation of propan -1- amine from butane nitrile.
Butane nitrile treated with acid hydrolysis followed by Hoffmann’s bromamide degradation. gives propan – 1 – amine.
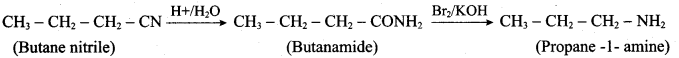
2. Preparation of propan – 1 – amine from propanamide.
When propanamide is treated with LiAIH4 in the presence of water gives propan – 1 – amine.
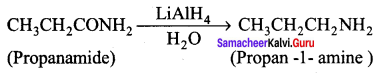
3. Preparation of propan – 1 – amine from 1 – nitropropane.
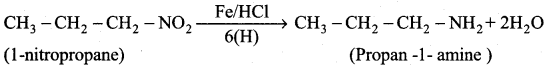
Reduction of 1 – Nitropropane using H2 / Ni or Fe / HCl gives propan – 1 – amine.
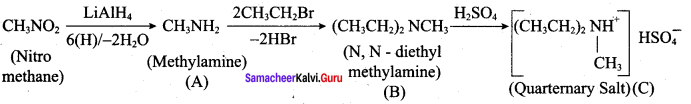
Question 11.
Identify A,B,C and D
![]()
Answer:
Question 12.
How will you convert dlethylamine into
- N, N – dlethylacetamide
- N – nitrosodiethylamine
Answer:
1. Conversion of diethylamine into N, N – diethylacetamide.

Diethylamine react with acetyichioride in the presence of pyridine to form N, N – diethyl acetamide.
2. Conversion of diethylamine into N – nitrosodiethylamine.

Question 13.
Identify A,B and C
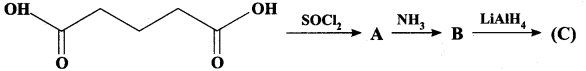
Answer:
Question 14.
Identify A, B, C and D
![]()
Answer:
Question 15.
Complete the following reaction
Answer:
Question 16.
Predict A, B, C and D for the follwing reaction.
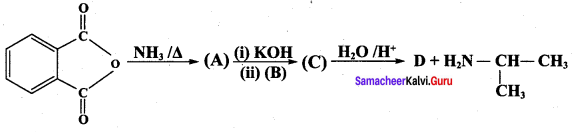
Answer:
Question 17.
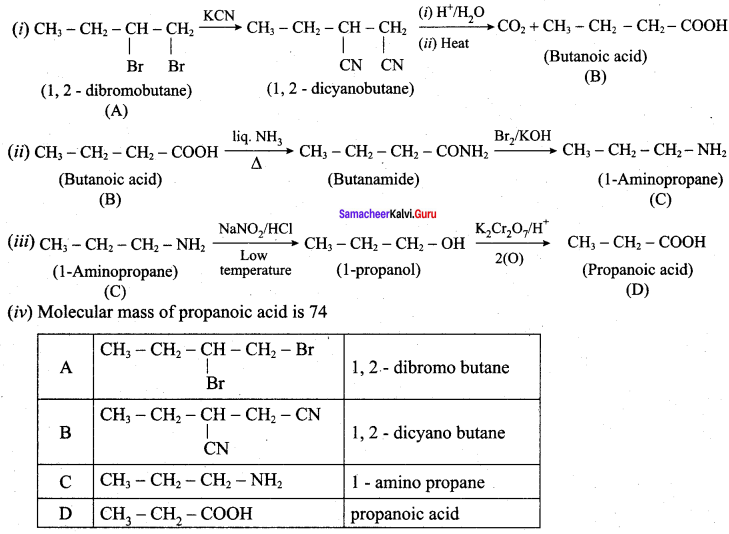
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with liberation of CO2. (B) on heating with liquid ammonia followed by treating with Br2 / KOH gives (C) which on treating with NaNO2 and HCI at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
Answer:
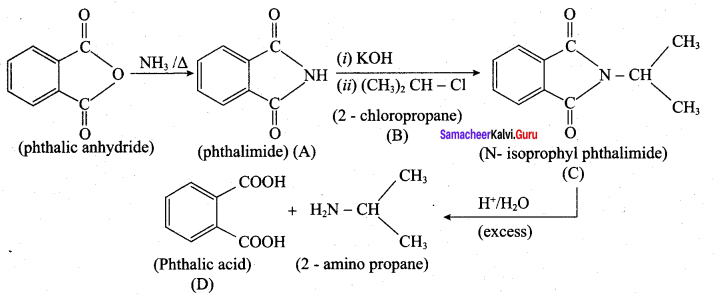
Question 18.
Identify A to E in the following frequncy of reactions.
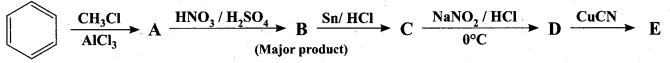
Answer:
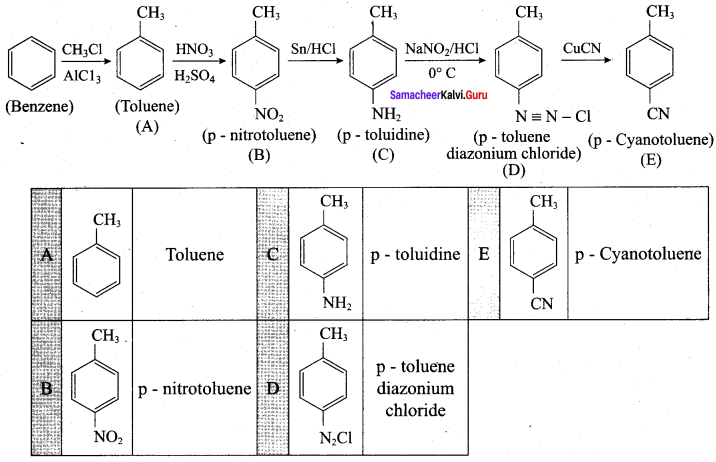
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds Evaluate Yourself
Question 1.
Write all possible isomers for the following compounds.
- C2H5 – NO2
- C3H7 – NO2
Answer:
1. Possible isomers for C2H5NO2 as following
(a) CH3 – CH2 – NO2 – Nitroethane
(b) CH3 – CH2 – O – N = O – Ethyl nitrite
(c)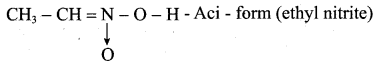
(d) H2N – CH2 – COOH – Glycine (amino acid)
(e) 
(f) 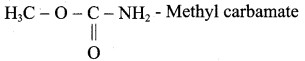
(g) 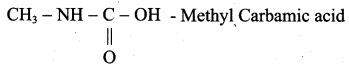
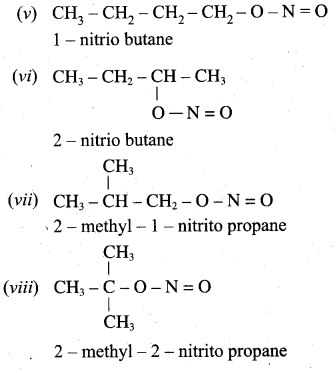
2. Possible isomers for C3H7NO2 as follows.
(a) CH3 – CH2 – CH2 – NO2 – 1 – Nitropropane
(b) CH3 – CH2 – CH2 – O – N = O – propane – 1 – nitrite
(c) 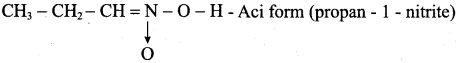
(d) 
(e) 
(f) H2N – CH2 – CH2 – COOH – Alanine
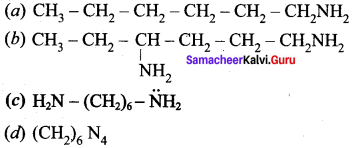
Question 2.
Find out the product of the following reactions
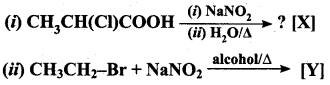
Answer:
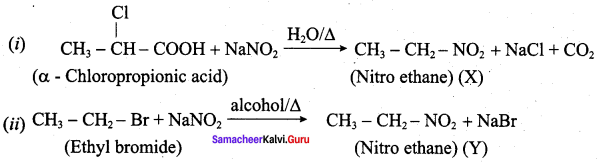
Question 3.
Predict the major product that would be obtained on nitration of the following compounds.
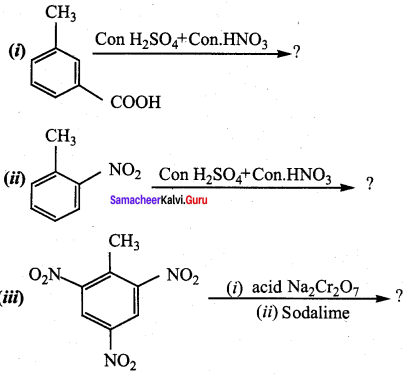
Answer:
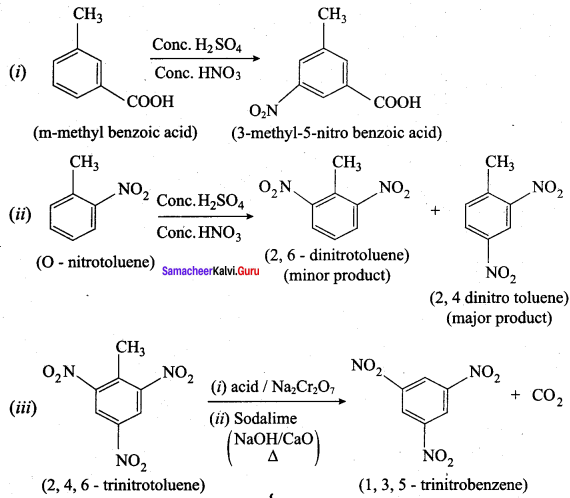
Question 4.
Draw the structure of the following compounds
- Neopentylamine
- Tert – butylamine
- α – amino propionaldehyde
- tribenzylamine
- N – ethyl – N – methylhexan – 3 – amine
Answer:
1. Neopentylamine:
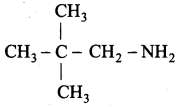
2. Tert – butylamine
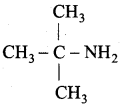
3. α – amino propionaldehyde
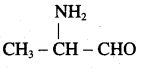
4. tribenzylamine
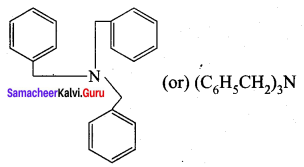
5. N – ethyl – N – methylhexan – 3 – amine
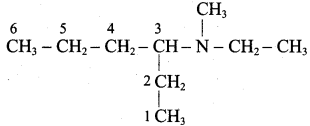
Question 5.
Give the correct IUPAC names for the following amines.
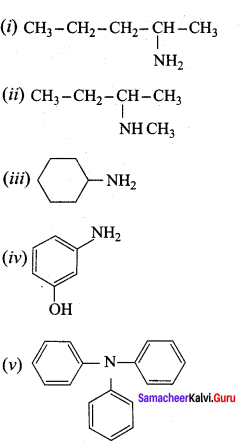
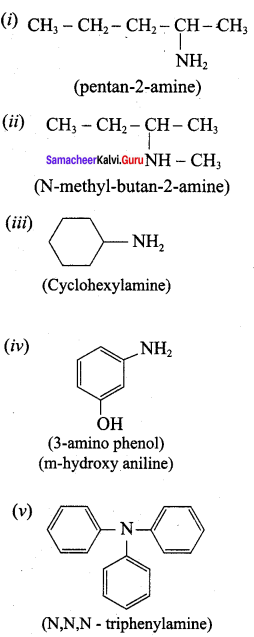
Answer:
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds Additional Question
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds 1 Mark Questions and Answers
I. Choose the correct answer.
Quesiton 1.
Which one of the following play an important role in bioregulation and neurotransmission?
(a) Acid derivatives
(b) Carbonyl compounds
(c) Organic derivatives of ammonia
(d) Aromatic hydro carbons
Answer:
(c) Organic derivatives of ammonia
Question 2.
Which of the following is needed to maintain the health of nerves, skin and red blood cells?
(a) Vitamin B12
(b) Vitamin B6
(c) Vitamin B1
(d) Vitamin C
Answer:
(b) Vitamin B6
Question 3.
Which one of the following is needed to maintain the health of nerves and skin?
(a) Pyridoxine
(b) Cobalamine
(c) Dopamine
(d) Histamine
Answer:
(a) Pyridoxine
Question 4.
Which one of the following is act as neurotransmitter?
(a) Pyridoxine
(b) Histamine
(c) Dopamine
(d) Cyano cobalamine
Answer:
(c) Dopamine
Question 5.
Which one of the following dilates blood vessels?
(a) Histamine
(b) Streptomycin
(c) Penicillin
(d) Dopamine
Answer:
(a) Histamine
Question 6.
Which one of the following is an example of primary nitro alkane?
(a) 2 – nitropropane
(b) Ethyl nitrite
(c) Nitro ethane
(d) 2 – methyl – 2 – nitropropane
Answer:
(c) Nitro ethane
Question 7.
2 – methyl – 2 – nitropropane belongs to
(a) 1° nitro alkane
(b) 3° nitro alkane
(c) 2° nitro alkane
(d) nitro arenes
Answer:
(b) 3° nitro alkane
Question 8.
Which of the following is an example for 2° nitro alkane?
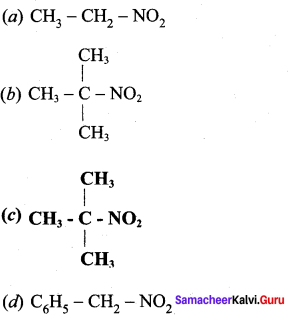
Answer:
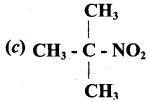
Question 9.
Which one of the following is an example for nitro arenes?
(a) C6H5 – CH2 NO6
(b) C6H5NH2
(c) CH3 – CH2 – O – NO
(d) C6H5NO2
Answer:
(d) C6H5NO2
Question 10.
The IUPAC name of
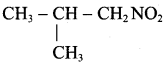 is ………………
is ………………
(a) 1 – nitro butane
(b) 2 – methyl – 1 – nitro propane
(c) Isobutyl nitrate
(d) 1 – Nitro iso butane
Answer:
(b) 2 – methyl – 1 – nitro propane
Question 11.
Which one of the following is the structure of 2, 2 – dimethyl – 1 – nitro propane?
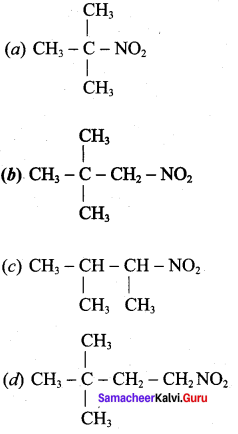
Answer:
Question 12.
1 – nitrobutane and 2 – methyl – 1 – mtropropane are belong to ……………..
(a) position isomerism
(b) functional isomerism
(c) Tautomerism
(d) chain isomerism
Answer:
(d) chain isomerism
Question 13.
Which of the following pair shows functional isomerism?
(a) 1 – nitro butane and 2 – nitro butane
(b) 1 – nitro butane and butyl nitrite
(c) 1 – nitro butane and 2 – methyl – 1 – nitropropane
(d) 2 – nitro butane and 2 – methyl – 2 – nitro propane
Answer:
(b) 1 – nitro butane and butyl nitrite
Question 14.
Which of the following pair shows position isomerism?
(a) 1 – nitro butane and butyl nitrite
(b) Nitro methane and methyl nitrite
(c) 1 – nitro butane and 2 – nitro butane
(d) 1 – nitro butane and 2 – methyl – 1 – nitro propane
Answer:
(c) 1 – nitro butane and 2 – nitro butane
Question 15.
Nitro methane and methyl nitrite are the examples of ……………
(a) Position isomerism
(b) chain isomerism
(c) metarnersm
(d) Tautomerism
Answer:
(d) Tautomerism
Question 16.
Consider the following statements.
(i) Nitro form of alkane dissolves in NaOH instantly
(ii) Nitro form of alkane decolourises FeCI3 solution
(iii) Nitro form of alkane are more acidic
Which of the above statement(s) is / are not correct?
(a) (i) only
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (ii) only
Answer:
(c) (i) and (iii)
Question 17.
Consider the following statements.
(i) Aci form of nitro alkanes dissolves in NaOH slowly.
(ii) Aci form of nitro alkane gives reddish brown colour with FeCl3
(iii) Aci form of nitro alkane’s electrical conductivity is low.
Which of the above statement(s) is / are correct?
(a) (ii) only
(b) (i) only
(c) (iii) only
(d) (i) and (iii)
Answer:
(a) (ii) only
Question 18.
Which one of the following does not exhibit tautomerism?
(a) 1 – nitro ethane
(b) Nitro methane
(c) methyl nitrite
(d) 2 – methyl – 2 – nitro propane
Answer:
(d) 2 – methyl – 2 – nitro propane
Question 19.
Identify the compound which does not exhibit tautomerism?
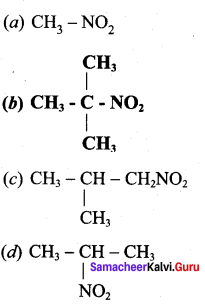
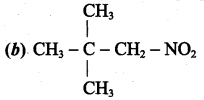
Answer:
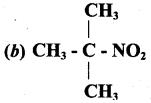
Question 20.
The correct decreasing order of acidity of nitro alkane is …………….
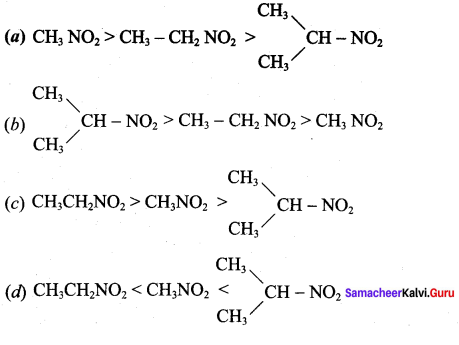
Answer:
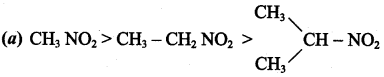
Question 21.
Which one of the following mechanism is followed by the reaction of Ethyl bromide with ethanolic solution of potassium nitrite ……………
(a) SN1
(b) SN2
(c) SNi
(d) E1
Answer:
(b) SN2
Question 22.
Which one of the following is formed when Ethyl bromide reacts with ethanolic solution of potassium nitrite?
(a) Nitro methane
(b) 2 – Nitro propane
(c) 1 – nitro propane
(d) nitro ethane
Answer:
(d) nitro ethane
Question 23.
Which method is used to separate the mixture of nitroalkane?
(a) Crystallization
(b) zone refining
(c) fractional distillation
(d) sublimation
Answer:
(c) fractional distillation
Question 24.
Which of the following is formed when ethane is heated with conc. HNO3 at 675 K?
(a) Nitro propane
(b) Nitro ethane
(c) Nitro methane
(d) both (ii) and (iii)
Answer:
(d) both (ii) and (iii)
Question 25.
What is the product formed when α – chioro acetic acid is boiled with aqueous solution of sodium nitrite?
(a) Nitro ethane
(b) Nitromethane
(c) Acetamide
(d) α – chloro acetamide
Answer:
(b) Nitromethane
Question 26.
Which one of the following reagent is used to convert teritary butylamine to tertiary nitro alkane?
(a) Aqueous KMnO4
(b) Cone HNO3
(c) Sn / HCI
(d) alcoholic KOH
Answer:
(a) Aqueous KMnO4
Question 27.
The reagent used in the conversion of acetaldoxime to nitro ethane (1°) is …………
(a) aqueous KMnO4
(b) trifluoro peroxy acetic acid
(c) alcoholic KOH
(d) Cone. HNO3
Answer:
(b) trifluoro peroxy acetic acid
Question 28.
Which of the following is called oil of mirbane?
(a) Nitro methane
(b) Nitro propane
(c) Nitro benzene
(d) Nitro ethane
Answer:
(c) Nitro benzene
Question 29.
On direct nitration of nitro benzene gives ………………
(a) 0 – dinitro benzene
(b) m – dinitro benzene
(c) p – dinitro benzene
(d) 2, 4, 6 – trinitrobenzene
Answer:
(b) m – dinitro benzene
Question 30.
Amino group can be directly converted into nitro group by ……………
(a) Caro’s acid
(b) Fuming mixture of conc. HNO3 + conc. H2SO4
(c) NaNO2 + HCI
(d) Ethanolic KNO2
Answer:
(a) Caro’s acid
Question 31.
Amino group can be directly converted into nitro group by ………….
(a) Caro’s acid
(b) marshall’s acid
(c) Peroxy trifluoro acetic acid
(d) all the above
Answer:
(d) all the above
Question 32.
Consider the following statements.
(i) Nitro alkanes have high points because of their highly polar nature
(ii) Alkyl nitrites have lower boiling points than nitro alkanes.
(iii) Nitro alkanes are readily soluble in water due to intermolecular hydrogen bonding formation.
Which of the above statement(s) is / are not correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i) and (ii)
Answer:
(c) (iii) only
Question 33.
The reagent used to convert Nitromethane to methyl amine is ……..
(a) Zn/NH4Cl
(b) Sn/HCI
(c) H2SO5
(d) H2S2O8
Answer:
(b) Sn/HCI
Question 34.
The reagent used to convert Nitromethane to N – methyl hydroxylamine is …………
(a) Sn/HCl
(b) Zn/NH4CI
(c) Ni
(d) Pd/BaSO4
Answer:
(b) Zn/NH4CI
Question 35.
Which one of the following is formed when ethyl nitrite is treated with Sn/HCl?
(a) Nitro ethane
(b) Ethylamine
(c) Ethyl alcohol
(d) Ethanamide
Answer:
(c) Ethyl alcohol
Question 36.
The product formed when nitro ehtane is boiled with conc. HCl is ……………
(a) Acetic acid
(b) Ethyl chloride
(c) Ethanoyl chloride
(d) Amino ehtane
Answer:
(a) Acetic acid
Question 37.
Which one of the following is formed when 2- nitro propane is boiled with conc.HCI?
(a) Ethanoic acid
(b) Propanoic acid
(c) Propanoyl chloride
(d) Acetone
Answer:
(d) Acetone
Question 38.
Which of the following does not react with conc. HCI?
(a) Nitro ethane
(b) 2 – methyl – 2 – nitropropane
(c) 2 – nitro propane
(d) Aniline
Answer:
(b) 2 – methyl – 2 – nitropropane
Question 39.
Acid (or) Basic hydrolysis of ethyl nitrite gives ………….
(a) Ethanoic acid
(b) Nitro ethane
(c) Ethanol
(d) Aceto nitrile
Answer:
(c) Ethanol
Question 40.
Which one of the following is formed when nitro methane reacts with chlorine and NaOH?
(a) CH3CI
(b) CH3COCl
(c) CCI3NO2
(d) CHCI2NO2
Answer:
(c) CCI3NO2
Question 41.
Which one of the following reagent is used to convert Nitrobenzene to aniline?
(a) Sn/HCI
(b) Zn/NH4CI
(c) Fe/H2O(g)
(d) Zn/NaOH
Answer:
(a) Sn/HCI
Question 42.
Which one of the following is the best reagent used to convert Nitrobenzene into Nitroso benzene?
(a) Sn/HCI
(b) Zn/NH4CI
(c) Fe/H2O(g)
(d) SnCl2 + KOH
Answer:
(c) Fe/H2O(g)
Question 43.
Identify the reagent used to convert Nitrobenzene into hydrazo benzene?
(a) Zn/NaOH
(b) Zn/NH4CI
(c) Sn/HCI
(d) SnCI2 + KOH
Answer:
(a) Zn/NaOH
Question 44.
Which one of the following is formed when nitrobenzene is treated with Fe/H2O(Steam)?
(a) Aniline
(b) Phenyl hydroxylamine
(c) Nitroso benzene
(d) Azobenzene
Answer:
(c) Nitroso benzene
Question 45.
Which one of the following is formed when nitrobenzene is treated with Zn/NaOH?
(a) Phenyl amine
(b) Phenyl hydroxylamine
(c) Azo benzene
(d) Hydrazo benzene
Answer:
(d) Hydrazo benzene
Question 46.
Which one of the following is formed when nitrobenzene is treated with SnCI2/KOH?
(a) Azo benzene
(b) Azoxy benzene
(c) Hydrazo benzene
(d) Nitroso benzene
Answer:
(a) Azo benzene
Question 47.
Which of the following is formed when nitro benzene undergoes electrolytic reduction?
(a) Aniline
(b) Phenyl hydroxylamine
(c) p – amino phenol
(d) all the above
Answer:
(d) all the above
Question 48.
Which of the following can be used to reduce nitrobenzene to aniline?
(a) LiAIH4
(b) Sn + HCI
(c) Pt/H2
(d) all the above
Answer:
(d) all the above
Question 49.
What will be the product formed when nitrobenzene is treated with conc. HNO3 and conc.H2SO4 at 373K?
(a) 1, 3 – dinitro benzene
(b) 1, 4 – dinitro benzene
(c) 1, 3, 5 – trinitro benzene
(d) all the above
Answer:
(a) 1, 3 – dinitro benzene
Question 50.
Which one of the following is formed when nitrobenzene is treated with cone. HNO3 and H2SO4 473 K?
(a) 1, 2 – din itro benzene
(b) 1, 4 – dintro benzene
(c) 1, 3 – dinitro benzene
(d) 1, 3, 5 – trinitro benzene
Answer:
(d) 1, 3, 5 – trinitro benzene
Question 51.
What will be the product formed when 1, 3, 5 – trinito toluene is treated with acidified Na2Cr2O7 and sodalime?
(a) TNB
(b) TNT
(c) TNG
(d) GTN
Answer:
(a) TNB
Question 52.
What is the IUPAC name of
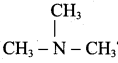
(a) Tertiary butyl amine
(b) Trimethyl amine
(c) N, N – dimethyl methanamine
(d) N – methyl ethanamine
Answer:
(c) N, N – dimethyl methanamine
Question 53.
Which one of the following is called (N – ethyl – N – methyl) propanamine?
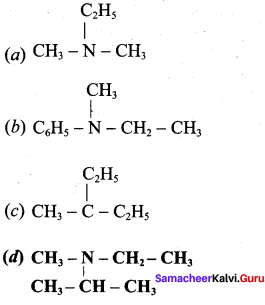
Answer:
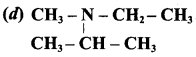
Question 54.
The IUPAC name of
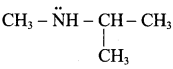 is ……………..
is ……………..
(a) Methyl iso propyl amine
(b) N – methyl propan – 1 – amine
(c) N, N – dimethyl methanamine
(d) propan – 1 – amine
Answer:
(b) N – methyl propan – 1 – amine
Question 55.
What is the IUPAC name of
(a) Ethyl methyl isopropylamine
(b) N, N – dimethyl methanamine
(c) N, N – diethyl butan – 1 – amine
(d) N – ethyl – N – methyl propan -2 – amine
Answer:
(d) N – ethyl – N – methyl propan -2 – amine
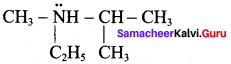
Question 56.
Which one of the following is called Hexane – 1, 6 – diamine?
Answer:
![]()
Question 57.
Which one of the following is the TUPAC name of CH2 = CH – CH2 – NH2?
(a) Isopropyl amine
(b) Allylamine
(c) 1 – amino propane
(d) prop – 2 – en – 1 – amine
Answer:
(d) prop – 2 – en – 1 – amine
Question 58.
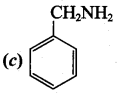
Which one of the following is the structure of phenyl methanamine?
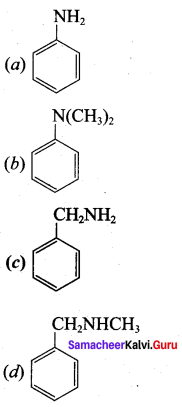
Answer:
Question 59.
Consider the following statements.
(i) Nitrogen atom of amines is trivalent and carries a lone pair of electron.
(ii) Nitrogen atom of amines is Sp2 hybridised.
(iii) Amines posses pyramidal geometry.
Which of the above statement(s) is / are correct?
(a) (i) and (ii)
(b) (i) and (iii)
(c) (iii) only
(d) (ii) only
Answer:
(b) (i) and (iii)
Question 60.
What is the C – N – C bond angle of trimethylamine?
(a) 109°. 5′
(b) 107°
(c) 108°
(d) 108°. 31’
Answer:
(c) 108°
Question 61.
Which one of the following is the geometry of amines?
(a) Tetrahedral
(b) Pyramidal
(c) Planar triangle
(d) square planar
Answer:
(b) Pyramidal
Question 62.
Which one of the following is formed when cyano methane reacts with LiAIH4?
(a) Ethanamine
(b) Methane
(c) Methanoic acid
(d) Acetic acid
Answer:
(a) Ethanamine
Question 63.
The reducing agent used in mendius reaction is …………
(a) H2/Ni
(b) LiAIH4
(c) Na/C2H5OH
(d) Sn/HCI
Answer:
(c) Na/C2H5OH
Question 64.
The product formed when methyl isocyanide is reduced by Na(Hg)/C2H5OH is …………
(a) Isopropyl amine
(b) Ethanamine
(c) N – methyl methanamine
(d) N – ethyl methanamine
Answer:
(c) N – methyl methanamine
Question 65.
Which one of the following is formed when acetamide reacts with LiAlH4 and H2O?
(a) Methyl amine
(b) Ethylamine
(c) Ammonium acetate
(d) N – methyl ethanamine
Answer:
(b) Ethylamine
Question 66.
In which reaction acetamide is changed to methylamine by the action of Br2/KOH?
(a) Gapriel phthalimide synthesis
(b) Hoffmann degration reaction
(c) Mendius reaction
(d) Mustard oil reaction
Answer:
(b) Hoffmann degration reaction
Question 67.
Which one of the reaction is used in the synthesis of aliphatic primary amines?
(a) Hoffmann ammonolysis
(b) Rosenmund’s reduction
(c) Carbylamine reaction
(d) Gabriel phthalimide synthesis
Answer:
(d) Gabriel phthalimide synthesis
Question 68.
The conversion of ethanol into all types of amines by the action of ammonia along with Alumina is …………
(a) HVZ reaction
(b) Sabatier – mailhe method
(c) Carbylamine reaction
(d) Mendius reaction
Answer:
(b) Sabatier – mailhe method
Question 69.
Consider the following statements.
(i) Amines have higher boiling point than alcohols.
(ii) Lower aliphatic amines are colourless gases whereas higher amines have fish like small.
(iii) Aniline and arylamines are colourless but when exposed to air they become coloured due to oxidation.
Which of the above statement(s) is / are correct?
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) only
(d) (iii) only
Answer:
(b) (ii) and (iii)
Question 70.
The correct order of basic strength in the case of ailcyl substituted amines is …………
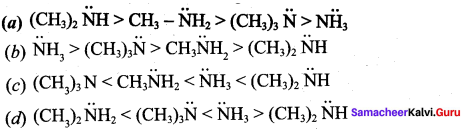
Answer:
![]()
Question 71.
The correct order of basic strength in the case of substituted ethyl amines is ………….
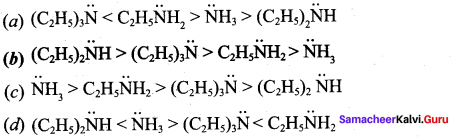
Answer:
![]()
Question 72.
The relative basicity of amine follows the order as ………….
(a) Alkyl amines > Aralkyl amines > Ammonia > N – aralkylamine > Arylamine
(b) Aralkyl amines > Ammonia > Arylamine > Alkyl amine > N – aralkylamine
(c) Arylamine Alkyl amine N – aralkylanilne
(d) N – aralkylamine < Arylamine < Ammonia < Alkyl amine < Aralkyl amine Answer: (a) Alkyl amines > Aralkyl amines > Ammonia > N – aralkylamine > Arylamine
Answer:
(a) Alkyl amines > Aralkyl amines > Ammonia > N – aralkylamine > Arylamine
Question 73.
Identify the name of the reaction in which aniline reacts with Benzoyl chloride to form N – Phenyl benzamide?
(a) Hoffmann degradation reaction
(b) Gabriel phthalimide synthesis
(c) Schotten – Baumann reaction
(d) Mustard oil reaction
Answer:
(c) Schotten – Baumann reaction
Question 74.
Which one of the product is formed when aniline reacts with benzoyl chloride in the presence of NaOH?
(a) N – Phenyl benzamide
(b) N – Phenyl ethanamide
(c) Benzamide
(d) N – Benzyl aniline
Answer:
(b) N – Phenyl ethanamide
Question 75.
Which one of the following is formed as product when ethylamine reacts with nitrous acid?
(a) Ethyl nitrite
(b) Nitro ethane
(c) Ethanol
(d) Ethane nitrile
Answer:
(c) Ethanol
Question 76.
Identify X in the following reaction?
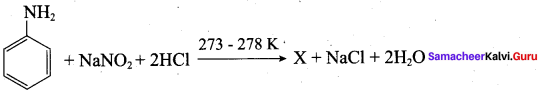
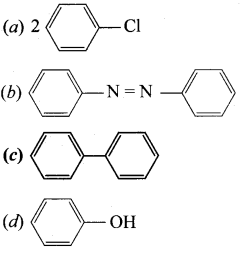
Answer:
Question 77.
The reaction of aniline with nitrous acid at low temperature is known as …………
(a) Carbylamme reaction
(b) mustard oil reaction
(c) Diazotisation
(d) Sand meyer’s reaction
Answer:
(c) Diazotisation
Question 78.
Which one of the product is formed with N-methyl aniline reacts with nitrous acid?
(a) Anilinium chloride
(b) N – nitroso methyl phenylamine
(c) Benzene diazonium chloride
(d) Benzylamine
Answer:
(b) N – nitroso methyl phenylamine
Question 79.
Which one of the réaction is called Libermann’s nitroso set?
(a) N – methyl aniline Conc. ![]() N – nitroso methyl phenylamine.
N – nitroso methyl phenylamine.
(b) ![]() Benzene diazonium chloride.
Benzene diazonium chloride.
(c) Aniline + CHCI3 + 3KOH → Phenyl iso cyanide.
(d) Methyl amine + CHCl3 + 3KOH → Methyl iso cyanide.
Answer:
(a) N – methyl aniline Conc. ![]() N – nitroso methyl phenylamine.
N – nitroso methyl phenylamine.
Question 80.
The conversion of N – methyl aniline into N – nitrosomethyl phenyl amine is known as …………
(a) Carbylamine reaction
(b) mustard oil reaction
(c) Diazotisation
(d) Libermann’s nitroso test
Answer:
(d) Libermann’s nitroso test
Question 81.
Which one of the following reaction is used to identify primary amines?
(a) Schotten – Baumann reaction
(b) Carbylamine reaction
(c) Sand meyer’s reaction
(d) Gattermann reaction
Answer:
(b) Carbylamine reaction
Question 82.
The reaction between methylamine and CS2 is known as ……………
(a) mustard oil reaction
(b) Carbylamine reaction
(c) Sand meyer’s reaction
(d) Gabriel phthalirnide synthesis
Answer:
(a) mustard oil reaction
Question 83.
Which one of the following is formed when aniline reacts with CS2 followed by hydrolysis by cone. HCI?
(a) Phenyl isocyanide
(b) phenyl cyanide
(c) Phenvi isothio cyanate
(d) Benzene diazonium chloride
Answer:
(c) Phenvi isothio cyanate
Question 84.
Consider the following reaction.
(i) Aniline does not undergo friedel – crafts reaction
(ii) Aromatic amine on treatment with NaNO2 + HCI gives diazonium slats.
(iii) Aniline is more basic than ammonia.
Which of the above statement(s) is / are not correct?
(a) (i) only
(b) (i) and (ii)
(c) (iii) only
(d) (ii) only
Answer:
(c) (iii) only
Question 85.
Which one of the following is formed when aniline reacts with cone. H2SO4?
(a) Zwitter ion
(b) Acetanilide
(c) Suiphanilic acid
(d) p – sulphonic bcnzoic acid
Answer:
(c) Suiphanilic acid
Question 86.
Consider the following statements.
(i) Benzene diazonium chloride aqueous solution are neutral to litmus.
(ii) The stability of arene diazonium salt is due to the dispersal of the positive charge over the ring.
(iii) Benzenediazonium chloride is reddish brown colour liquid.
Which of the above statement(s) is / are correct?
(a) (iii) and (ii)
(b) (i) and (ii)
(c) (i) and (iii)
(d) (iii) only
Answer:
(b) (i) and (ii)
Question 87.
Identify the product formed when Benzene diazonium chloride reacts with phosphinic acid?
(a) Benzene
(b) Chioro benzene
(c) Phenol
(d) cyano benzene
Answer:
(a) Benzene
Question 88.
The conversion of Benzene diazonium chloride into chlorobenzene is known as …………
(a) Gabriel phthalimide synthesis
(b) Carbylamine reaction
(c) Sand meyer reaction
(d) Coupling reaction
Answer:
(c) Sand meyer reaction
Question 89.
Identify X and Y in the following reaction
![]()
(a) C6H5CI + N2
(b) C6H6 + N2
(c) C6H5CI + NH4CI
(d) C6H5CI + H2
Answer:
(a) C6H5CI + N2
Question 90.
Which one of the following is formed when benzene diazonium chloride is boiled with water?
(a) Benzene
(b) Phenol
(c) Chiorobenzene
(d) Aniline
Answer:
(b) Phenol
Question 91.
Complete the following reaction.
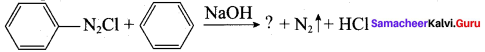
Answer:
Question 92.
What is the name of the reaction in which benzene diazonium chloride react with benzene to give Biphenyl?
(a) Sandmeyer’s reaction
(b) Gomberg reaction
(c) Gattermann reaction
(d) Baltz – schiemann reaction
Answer:
(b) Gomberg reaction
Question 93.
Which one of the following reagent reacts with ben.zene diazonium chloride to give biphenyl?
(a) Chioro benzene
(b) Bromobenzene
(c) Benzene
(d) Acetic acid
Answer:
(c) Benzene
Question 94.
Which one of the following is formed when Benzene dia.zonium chloride reacts with Aniline?
(a) p – hydroxy azo benzene
(b) 2 – phenyl azo – 4 – methyl
(c) Biphenyl
(d) p – amino azo benzene
Answer:
(d) p – amino azo benzene
Question 95.
Which one of the following should react with Benzene diazonium chloride to get orange dye?
(a) Aniline
(b) phenol
(c) 0 – cresol
(d) P – cresol
Answer:
(b) phenol
Question 96.
Aniline + Benzene diazonium chloride → X. Identify X.
(a) orange dye
(b) yellow dye
(c) malachite green dye
(d) madder dye
Answer:
(b) yellow dye
Question 97.
Which one of the following is the IUPAC name of CH3 – CH2 – CH2CN?
(a) Propiono nitrite
(b) Butane cyanide
(c) Isobutyro nitnie
(d) Butane nitrile
Answer:
(d) Butane nitrile
Question 98.
Which one of the following is formed when methyl magnesium bromide reacts with cyanogen chloride?
(a) methane nitrile
(b) ethane nitrite
(c) Acetamide
(d) Nitro ethane
Answer:
(b) ethane nitrite
Question 99.
The reagent used in the conversion of CH3CONH2 into CH3CN is …………
(a) Br2/KOH
(b) conc. HNO3
(c) Sn/HCI
(d) P2O5
Answer:
(d) P2O5
Question 100.
The product of complete hydrolysis of Ethane nitrite is …………
(a) Ethane
(b) Ethyl nitrate
(c) Nitro ethane
(d) Acetic acid
Answer:
(d) Acetic acid
Question 101.
What is the name of the reaction that take place between Ethane nitrile and Ethyl propionate?
(a) Coupling reaction
(b) Levine and hauser acetylation
(c) Diazotisation
(d) Acetic acid
Answer:
(b) Levine and hauser acetylation
Question 102.
Which one of the following is formed when methyl iso cyanide ùndergoes acid hydrolysis?
(a) Dimethyl amine + H2O
(b) Acetic acid + Formic acid
(c) Methyl amine + Formic acid
(d) Methyl cyanide + Ammonia
Answer:
(c) Methyl amine + Formic acid
Question 103.
The product formed when methyl isocyanide is heated to 250°C is …………
(a) Methyl amine
(b) Methyl cyanide
(c) Ethyl Cyanide
(d) Amino ethane
Answer:
(b) Methyl cyanide
Question 104.
Which one of the following is used a fuel for cars?
(a) CH3NO2
(b) CH3NH2
(c) CH3N
(d) CH3NC
Answer:
(a) CH3NO2
Question 105.
Chloropicrin is used as ……….
(a) antiseptic
(b) analgesic
(c) insecticide
(d) fertilizer
Answer:
(c) insecticide
Question 106.
Which one of the following is used as a fuel additive and precursor to explosive?
(a) Nitroglycerine
(b) Nitro methane
(c) Nitro benzene
(d) Nitro ethane
Answer:
(d) Nitro ethane
Question 107.
Which one of the following is known as sweet spirit of nitre?
(a) 10 % solution of methyl nitrite
(b) 4% solution of ethyl nitrite
(c) 10% solution of ethyl nitrite
(d) 40% solution of methanal
Answer:
(b) 4% solution of ethyl nitrite
Question 108.
Which one of the following is used as diuretic?
(a) Nitro methane
(b) Nitrobenzene
(c) ethyl nitrite
(d) Oil of mirbane
Answer:
(c) ethyl nitrite
Question 109.
Which of the following is used to produce lubircating oils in motors and machinery?
(a) Nitro benzene
(b) m – dinitro benzene
(c) 1, 3, 5 – trinitro benzene
(d) Nitro glycerine
Answer:
(a) Nitro benzene
Question 110.
Which of the following is used in the manufacture of aniline, synthetic rubber, dyes and explosives like TNT, TNB?
(a) Nitro ethane
(b) Aminobenzene
(c) Nitro benzene
(d) Benzene diazonium chloride
Answer:
(c) Nitro benzene
Question 111.
Which of the following is used in textile industries and also as a solvent in perfume industries?
(a) Alkyl cyanide
(b) Alkyl iso cyanide
(c) Alkyl iso thio cyanate
(d) Alkyl amine
Answer:
(a) Alkyl cyanide
Question 112.
Which one of the following is used as an anticancer agent used to stomach and colon cancer?
(a) Vitamin C
(b) Cobalamine
(c) mitomycin C
(d) Streptomycin
Answer:
(c) mitomycin C
Question 113.
In the Hoffmann bromamide degradation reaction, the number of moles of KOH and Br2 used per mole of amine produced are …………..
(a) four moles of KOH and two moles of Br2
(b) two moles of KOH and two moles of Br2
(c) four moles of KOH and one moles of Br2
(d) one moles of KOH and one moles of Br2
Answer:
(c) four moles of KOH and one moles of Br2
Question 114.
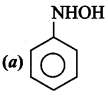
What is the product obtained in the following reaction?
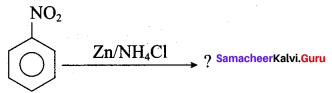
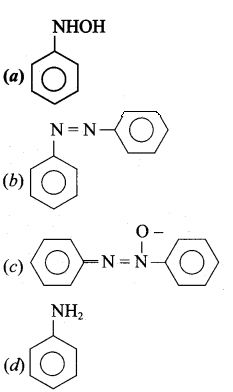
Answer:
Question 115.
The reagent with which the following reaction is best accomplished is ………….
(a) H3PO3
(b) H3PO4
(c) H3PO2
(d) NaHSO3
Answer:
(c) H3PO2
Question 116.
The amine “A” when treated with nitrous acid gives yellow oily substance. The amine “A” is …………
(a) Triethylamine
(b) Trimethylamine
(c) aniline
(d) Ethyl methyl amine
Answer:
(d) Ethyl methyl amine
Question 117.
Which one of the following amide will not undergo Hoffmann bromamide reaction?
(a) CH3CONH2
(b) CH3CONHCH3
(c) C6H5CONH2
(d) CH3CH2CONH2
Answer:
(b) CH3CONHCH3
Question 118.
Replacement of diazonium group by fluorine is known as …………..
(a) Gattennann reaction
(b) Sandmeyer reaction
(c) Baltz – Schiemann reaction
(d) Comberg reaction
Answer:
(c) Baltz – Schiemann reaction
Question 119.
Considering the basic strength of amines in aqueous solution, which are has the smallest pK value?
(a) CH3NH2
(b) (CH3)3N
(c) C6H5NH2
(d) (CH3)3NH
Answer:
(d) (CH3)3NH
Question 120.
Which one of the following is the strongest base in aqueous solution?
(a) Trimethyl amine
(c) Dimethyl amine
(d) methyl amine
(b) Aniline
Answer:
(c) Dimethyl amine
Question 121.
Diethyl amine when treated with nitrous acid yields
(a) Diethyl ammonium nitrite
(b) Ethyl alcohol
(c) N – nitroso diethyl amine
(d) Triethyl ammonium nitrate
Answer:
(c) N – nitroso diethyl amine
Question 122.
Which one of the following on reduction with Lithium aluminium hydride yields a secondary amine?
(a) Methyl iso cyanide
(b) Acetamide
(c) Methyl cyanide
(d) Nitro ethane
Answer:
(a) Methyl iso cyanide
Question 123.
The action of nitrous acid on ethylamine gives
(a) Ethane
(b) ammonia
(c) Ethyl alcohol
(d) Ethyl nitrite
Answer:
(c) Ethyl alcohol
Question 124.
Indicate which nitrogen compound amongst the following would undergo Hoffmann reaction with Br2 and strong KOH to furnish primary amine kNH2.
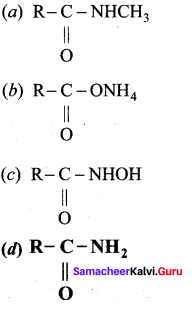
Answer:
Question 125.
The correct order of basicity of the following compounds is …………..
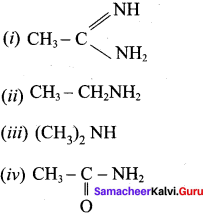
(a) (ii) > (i) > (iii) > (iv)
(b) (i) > (iii) > (ii) > (iv)
(c) (iii) > (i) > (ii) > (iv)
(d) (i) > (ii) > (iii) > (iv)
Answer:
(b) (i) > (iii) > (ii) > (iv)
Question 126.
Which of the following would be most reactive towards nitration?
(a) Benzene
(b) nitrobenzene
(c) Toluene
(d) Chiorobenzene
Answer:
(b) nitrobenzene
Question 127.
Aniline reacts with acetaldehyde to form.
(a) Schiff’s base
(b) carbylamine
(c) Imine
(d) acetaldoxime
Answer:
(a) Schiff’s base
Question 128.
Which of the following is the strongest base?
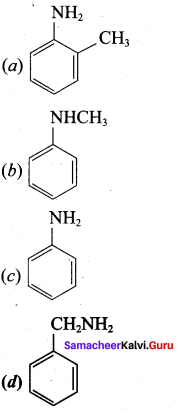
Answer:
Question 129.
A primary amine is formed from an amide by the treatment of bromine and alkali. The primary amine has ……………
(a) 1 Carbon atom less than amide
(b) 1 carbon atom more than amide
(c) 1 hydrogen atom less than amide
(d) 1 Hydrogen atom more than amide
Answer:
(a) 1 Carbon atom less than amide
Question 130.
Liebermann’s nitroso reaction is used for testing ………….
(a) 1° amine
(b) 2° amine
(c) 3° amine
(d) all the above
Answer:
(b) 2° amine
Question 131.
A nauseating smell in the carbylamine test for primary amines is due to the formation of …………
(a) iso cyanide
(b) chloroform
(c) cyanide
(d) iso thiocyanate
Answer:
(a) iso cyanide
Question 132.
A positive carbylamine test is given by …………
(a) N, N – dimethyl aniline
(b) 2, 4 – dimethyl aniline
(c) N – methyl – 0 – methyl aniline
(d) p – methyl benzylamine
Answer:
(b) 2, 4 – dimethyl aniline
Question 133.
When primary amine is heated with CS2 in the presence of excess of mercuric chloride, it gives isothiocyanate. This reaction is called ……………..
(a) Hoffmann bromamide reaction
(b) Carbylamine reaction
(c) Perkin’s reaction
(d) Hoffmann mustard oil reaction
Answer:
(d) Hoffmann mustard oil reaction
Question 134.
Diazo – coupling reaction is useful to prepare some
(a) Dyes
(b) proteins
(c) pesticides
(d) plastics
Answer:
(a) Dyes
Question 135.
Carbylamine test is used in the detection of …………
(a) aliphatic 2° amine
(b) Aromatic 1° amine
(c) Aliphatic l amine
(d) both aliphatic and aromatic 1° amine
Answer:
(d) both aliphatic and aromatic 1° amine
Question 136.
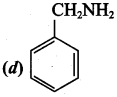
Which of the following amine will not react with nitrous acid to give nitrogen?
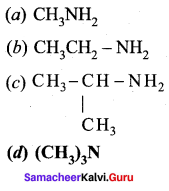
Answer:
![]()
Question 137.
Which of the following compound is expected to be more basic?
(a) Aniline
(b) Methylamine
(c) Ethylamine
(d) Hydroxylamine
Answer:
(c) Ethylamine
Question 138.
Nitro group in Nitro benzene is a ………….
(a) ortho directing group
(b) Meta directing group
(c) Para directing group
(d) ortho and para directing group
Answer:
(b) Meta directing group
Question 139.
Which of the following amines would undergo diazotisation?
(a) CH3NH2
(b) C2H5NH2
(c) C6H5NH2
(d) (CH3)2NH
Answer:
(c) C6H5NH2
Question 140.
Primary amines can be distinguished from secondary and tertiary amines by reacting with.
(a) CHCI3 and alkali
(b) CH3I
(c) CHCl3 alone
(d) Zn dust
Answer:
(a) CHCI3 and alkali
Question 141.
A solution of methylamine …………..
(a) turns blue litmus red
(b) turns red litmus blue
(c) does not effect red or blue litmus
(d) Bleaches litmus
Answer:
(b) turns red litmus blue
Question 142.
Oil of mirbane is ………..
(a) Aniline
(b) Nitro ethane
(c) p – amino azo benzene
(d) Nitro benzene
Answer:
(d) Nitro benzene
Question 143.
Identify the product Z in the series of the reaction ………….
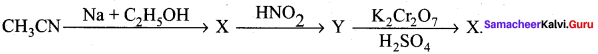
(a) CH3CHO
(b) CH3CONH2
(c) CH3COOH
(d) CH3CH2NHOH
Answer:
(c) CH3COOH
Solution:

Question 144.
Primary and secondary amines are distinguished by ……….
(a) Br2 / KOH
(b) HCIO4
(c) NH3
(d) HNO2
Answer:
(d) HNO2
Question 145.
Aniline on treatment with excess bromine water gives
(a) Anilinium bromide
(b) O – bromo aniline
(c) 2, 4, 6 – tribromo aniline
(d) p – bromo aniline
Answer:
(c) 2, 4, 6 – tribromo aniline
Question 146.
Which of the following is not used as an explosive?
(a) Trinitro toluene
(b) Trinitro benzene
(c) Trinitro glycerine
(d) Nitro benzene
Answer:
(d) Nitro benzene
Question 147.
Which of the following has a pyramidal structure?
(a) Trimethyl amine
(b) Water
(c) Acetylene
(d) Methane
Answer:
(a) Trimethyl amine
Question 148.
Which one of the following reacts with benzaldehyde to give schiff’s base.
(a) Acidified K2Cr2O7
(b) formaldehyde
(c) Aniline
(d) Potassium cyanide
Answer:
(c) Aniline
Question 149.
Ethyl amine can be prepared by the action of bromine and caustic potash on
(a) Acetamide
(b) propionamide
(c) Formamide
(d) Methyl Eyanide
Answer:
(b) propionamide
Question 150.
Which of the following reaction will not give primary amine?
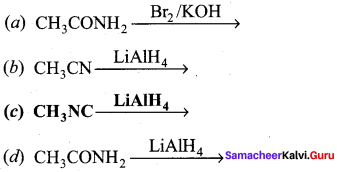
Answer:
![]()
Question 151.
Which of the following compound is the strongest base?
(a) Ammonia
(b) Aniline
(c) Methyl amine
(d) N – methyl aniline
Answer:
(c) Methyl amine
Question 152.
Azo dyes are prepared from ………….
(a) Phenol
(b) Aniline
(c) Benzaldehyde
(d) Both (i) and (ii)
Answer:
(d) Both (i) and (ii)
II. Fill in the blanks.
- ………….. is an organic compound needed to maintain the health of nerves, skin and red blood cells.
- Plants synthesis and to protect them from being eaten away by insect ………….. and ………….. other animals.
- ………….. compounds are the important constituents of explosives, drugs, dyes, fuels, polymers, synthetic rubbers.
- Dopamine act as …………..
- ………….. dilates blood vessels.
- Tertiary nitro alkanes donot exhibit ………….. due to the absence of a-H atom.
- Aci form of nitro alkanes gives ………….. colour with ferric chloride.
- Aci form of nitro alkanes are otherwise called ………….. or …………..
- Laboratory preparation of Nitro ethane from ethyl bromide follows ………….. mechanism
- Except ………….. other alkanes gives a mixture of nitro alkanes due to C – C cleavage by nitration of alkanes.
- Oxidation of acetaldoxime with ………….. gives 1 – nitro ethane.
- ………….. is suspected to cause genetic damage and be harmful to the nervous system.
- Nitro benzene on reduction with SnCl2 + KOH gives …………..
- Nitrobenzene on alkaline medium reduction gives …………..
- Amines posses ………….. geometry.
- The nitrogen atom in amine is ………….. hybridised.
- Gabriel phthalimide synthesis is used for the preparation of …………..
- Ammonolysis of hydroxyl compounds is called ………….. reaction.
- Aniline when exposed to air becomes coloured due to …………..
- Alkyl amines are stronger base than …………..
- Acylation and benzoylation of Aniline aer ………….. reactions.
- Libermann’s nitroso test is used to detect …………..
- ………….. test is used to identify primary amine.
- Direct nitration of aniline gives O and P – nitro aniline along with ………….. due to oxidation.
- The conversion of benzene diazonium chloride to benzene by H3PO2 proceeds through ………….. mechanism.
- Benzene diazonium chloride when boiled with water gives …………..
- The conversion of Benzene diazonium chloride is Biphenyl is called ………….. reaction.
- Coupling reaction generally occurs at ………….. position of Benzene ring.
- The condensation reaction of esters with nitrites containing a – hydrogen is known as …………..
- Chloropicrin is used as an …………..
- 4% solution of ethyl nitrite in alcohol is known as …………..
- Sweet spirit of nitre is used as …………..
- ………….. is used to produce lubricating oils in motors and machinery.
- ………….. an anti cancer agent used to treat stomach and colon cancer.
- Mitomycin C contains an ………….. ring.
- ………….. is used as percursor to explosive.
- An organic nitrogen compound ………….. is used as an insecticide.
- ………….. is known as sweet spirit of nitre.
- Chloropicnn ………….. is used as an insecticide.
Answer:
- Pyridoxine, vitamin B6
- alkaloids, biologically active amines
- Nitrogen
- Neurotransmitter
- Histamine
- tautomerism
- Reddish brown
- Pseudo acids (or) Nitronic acids
- SN2
- Methane
- trifluoroperoxy acetic acid
- Nitro ethane
- Azobenzene
- Hydrazobenzene
- Pyramidal
- SP3
- Aliphatic primary amines
- Sabatier – mailhe
- Oxidation
- Ammonia
- Nucleophilic substitution (or) Schotten Baumann
- Secondary amine
- Carbylamine reaction (or) Hoffmann mustard oil reaction
- dark coloured tars
- Free radical chain
- Phenol
- Gomberg
- Para
- Levine and hauser
- insecticide
- Sweet spirit of nitre
- diuretic
- Nitrobenzene
- Mitomycin C
- Aziridine
- Nitro ethane
- chloropicrm
- 4% solution of ethyl nifrite in alcohol
- CCI3NO2
III. Match the Column I and II using the code given below the columns.
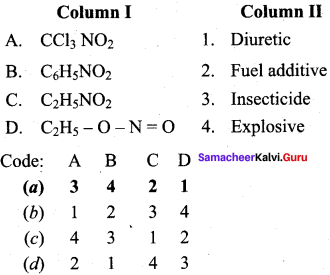
Question 1.
Answer:
(a) 3 4 2 1
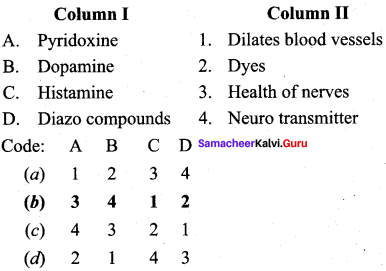
Question 2.
Answer:
(b) 3 4 1 2
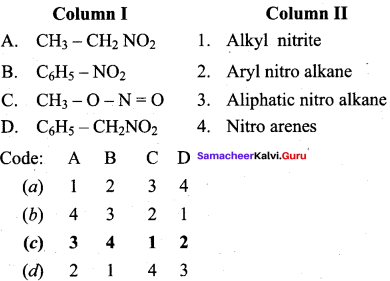
Question 3.
Answer:
(c) 3 4 1 2
Question 4.
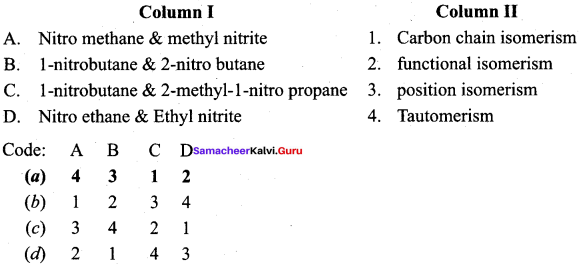
Answer:
(a) 4 3 1 2
Question 5.
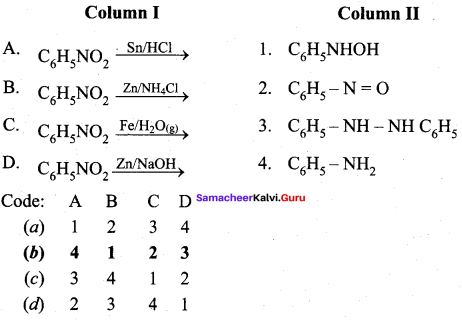
Answer:
(b) 4 1 2 3
Question 6.
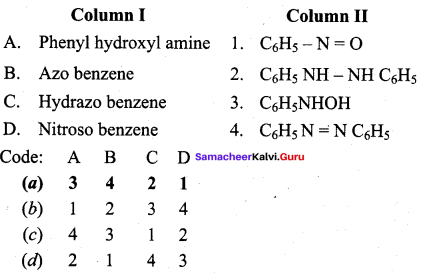
Answer:
(a) 3 4 2 1
Question 7.
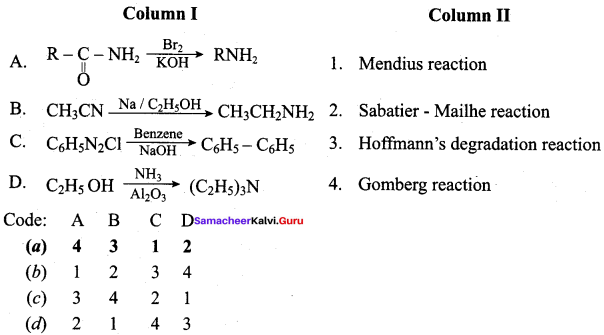
Answer:
(a) 3 1 4 2
Question 8.
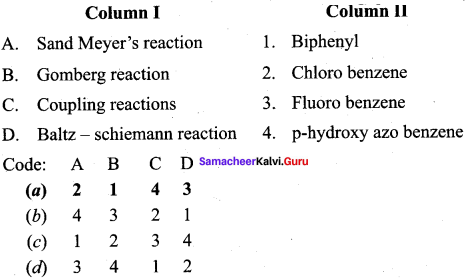
Answer:
(a) 2 1 4 3
Question 9.
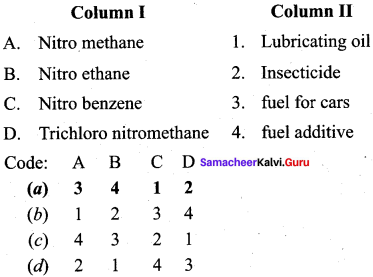
Answer:
(a) 3 4 1 2
Question 10.
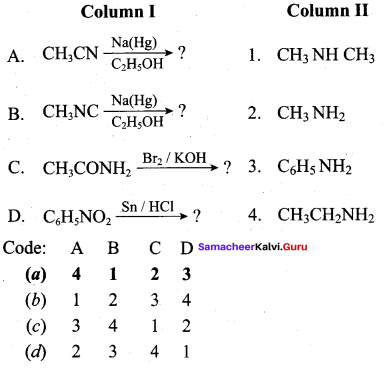
Answer:
(a) 4 1 2 3
IV. Assertion and reasons.
Question 1.
Assertion(A): Tertiary nitro alkanes do not exhibit tautomerism.
Reason (R): Tertiary nitro alkanes do not have of α – H atom.
(a) Both A and R are correct and R is explains A
(b) Both A and R are not correct
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is explains A
Question 2.
Assertion(A): Primary and secondary nitroalkanes show an equilibrium mixture of two tautomers namely nitro and aci form.
Reason (R): Both primary and secondary nitroalkanes are having a-H atoms.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of ofA
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 3.
Assertion(A): Nitro alkanes dissolve in NaOH solution to form a salt.
Reason (R): The α – H atom of 1° and 2° nitroalkanes show acidic character because of the electron withdrawing effect of NO2 group.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 4.
Assertion(A): 2 – nitro propane is more acidic than nitro mehtane.
Reason (R): When the number of alkyl group attached to a carbon increases, acidity decreases. due to +1 effect of alkyl groups.
(a) Both A and R are correct but R is not the correct explanation of of A
(b) Both A and R are correct and R is the correct explanation of A
(c) A is correct but R is wrong.
(d) A is wrong but R is correct
Answer:
(d) A is wrong but R is correct
Question 5.
Assertion(A): Nitrobenzene cannot be prepared from bromo benzene by action of ethanolic solution of potassium nitrite.
Reason (R): The bromine directly attached to the benzene ring cannot be cleaved easily.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 6.
Assertion(A): Nitrobenzene undergoes friedel – craft reaction.
Reason (R): Nitrobenzene have strong deactivating – NO2 group.
(a) Both A and R arc correct but R is not the correct explanation of A
(b) Both A and R are correct and R is the correct explanation of A
(c) A is wrong but R ¡s correct
(d) A is correct but R is wrong
Answer:
(c) A is wrong but R ¡s correct
Question 7.
Assertion(A): Amines posses pyramidal geometry.
Reason (R): Nitrogen atoms of amines is trivalent and has four sp3 hybridised orbital. Three sp3 orbitais overlap with orbitais of hydrogen and four sp3 orbitais contain a lone pair of electrons.
(a) Both A and R are correct and R ¡s the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong.
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R ¡s the correct explanation of A
Question 8.
Assertion(A): The C – N – C bond angle of trimethyl amine is 108°.
Reason (R): The bond angle of C – N – C is due to the repulsion between the bulky methyl groups.
(a) Both A and R are wrong
(b) A is correct and R is wrong
(c) Both A and R are correct and R is the correct explanation of A
(d) Both A and R are correct but R is not the correct explanation of A
Answer:
(c) Both A and R are correct and R is the correct explanation of A
Question 9.
Assertion(A): Aniline cannot be prepared by Gabriel phthalimide synthesis.
Reason (R): Arylhalides do not undergo nucleophilic substitution with the anion formed by phthalimide
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 10.
Assertion(A): Amines have lower boiling point than alcohols.
Reason (R): Nitrogen has lower electronegative value than oxygen and hence the N – H bond is less polar than – OH bond.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 11.
Assertion(A): Tertiary methyl amine is less soluble in water than methyl amine.
Reason (R): Solubility decreases due to the increase in size of the hydrophobic alkyl group.
(a) Both A and R are wrong
(b) A is correct but R is wrong.
(c) Both A and R are correct and R is the correct explanation of A
(d) Both A and R are correct but R is not the correct explanation of A
Answer:
(c) Both A and R are correct and R is the correct explanation of A
Question 12.
Assertion(A): Aniline reacts with acids to form salts and also reacts with electrophiles.
Reason (R): The lone pair of electrons on nitrogen atom in amines makes them basic as well as nucleophilic.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 13.
Assertion(A): Alkyl amines are stronger base than Ammonia.
Reason (R): When a +I gorup like alkyl group is attached to nitrogen increases the electron density on the nitrogen which makes the electron pair readily available for protonation.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is wrong but R is correct
(d) A is correct but R is wrong
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 14.
Assertion(A): 2° amines are more basic.
Reason (R): Due to + I effect, steric effect and hydration effect cause 2° amines are more basic.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 15.
Assertion(A): Aromatic amines are less basic than ammonia.
Reason (R): The lone pair of electrons on nitrogen atom in aniline (aromatic amine) gets delocalised over the benzene ring and less available for protonation.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are wrong
(c) A is correct but R is wrong.
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 16.
Assertion(A): Electrophilic substituion in aniline take place at ortho and para position.
Reason (R): The – NH2 group is a strong activating group and lone pair of electrons on the nitrogen atom is in conjugation with benzene ring that increases electron density at ortho and para position.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is not the correct explanation of A
(c) A is correct but R is wrong.
(d) Both A and R are wrong
Answer:
(a) Both A and R are correct and R is the correct explanation of A
Question 17.
Assertion(A): Acylation of amines gives a mono substituted product whereas alkylation of amines gives polysubstituted product.
Reason (R): Acyl group sterically hindered the approach to further acyl group.
(a) Both A and R are wrong
(b) A is correct but R is wrong
(c) Both A and R are correct and R is the correct explanation of A
(d) Both A and R are correct but R is not the correct explanation of A
Answer:
(c) Both A and R are correct and R is the correct explanation of A
Question 18.
Assertion(A): Acetanilide is less basic than anime.
Reason (R): Acetylation of aniline results in the decrease of electron density on nitrogen.
(a) Both A and R are correct and R explains A.
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R explains A.
Question 19.
Assertion(A): Aromatic 1° amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Aryl halides undergo nucleophilic substitution with the anion formed by phthalimide.
(a) Both A and R are correct and R is explains A
(b) Both A and R are wrong
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(b) Both A and R are wrong
Question 20.
Assertion(A): Aniline does not undergo Friedel – Crafts reaction.
Reason (R): Aniline donates its lone pair of electrons to the Lewis acid AiCl3 to form an adduct which inhibits further electrophilic substitution reaction.
(a) Both A and R are correct and R is explains A.
(b) Both A and R are wrong
(c) A is correct but R is wrong.
(d) A is wrong but R is correct
Answer:
(a) Both A and R are correct and R is explains A.
V. Find the odd one out and give the reasons.
Question 1.
Pyridoxine, Dopamine, Histamine, Aspirin.
Answer:
Aspirin:
It is acid derivative and used as medicine whereas other are organic nitrogen compounds used in medicine.
Question 2.
Trinitro glycerine, Glyceryl triacetate, Trinitro benzene, Trinitro toluene
Answer:
Glyceryl triacetate:
It is a an ester of glycerol whereas others are organic nitrogen compounds used in making explosives.
Question 3.
N – methyl methanamine, N – methyl ethanamine, N – phenyl benzamide, N,N – dimethyl methanamine
Answer:
N,N – dimethyl methanamine: It ¡s an example of tertiary amine whereas others are secondary amine.
Question 4.
Propan – 2 – amine, N – ethyl – N – methyl, propan – 2 – amine, N,N – dimethyl methanamine, N, N – diethyl butan – 1 – amine
Answer:
Propan – 2 – amine:
It is a secondary amine whereas others are tertiary amines.
Question 5.
P – hydroxy azo benzene, Hydrazo benzene, P – amino azo benzene, 2 – Phenyl azo – methyl phenol
Answer:
Hydrazo benzene: It is not a dye whereas others are dyes.
Question 6.
Methyl ISo cyanide, Methyl cyanide, Acetic anhydride, Ethyl amine Nitro ethane
Answer:
Acetic anhydride:
It is an acid derivative whereas others are organic nitrogen compounds.
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds 2 Mark Questions and Answers
Question 1.
What is called nitro compound? Give one example.
Answer:
1. Ntiro compounds are considered as the derivatives of hydrocarbons. 1f one of the hydrogen atom of hydrocarbon is replaced by the – NO2 group, the resultant organic compound is called a ntiro compound.
2. E.g., CH3 – CH2 – NO2. Nitro ethane
Question 2.
Define Tautomerism. Give example. Why tertiary nitro alkanes do not ethibit tautomerism?
Answer:
1. Tautomerism is an isomerism in which the isomers change into one another with great ease of shifting of proton so that they exist together in equilibrium.

2. Tertiary nitro alkanes do not exhibit tautomerism due to absence of a-H atom.
Question 3.
Differentiate between nitro form and acid form of tautomerism of nitro methane.
Answer:
Nitro form
- Less acidic
- Dissolves in NaOH slowly.
- Decolourises FeCl3 solution.
- Electrical conductivity is low.
Aci form
- More acidic and also called pseudo acids (or) Nitrolic acids.
- Dissolves in NaOH instantly.
- With FeCI3 gives reddish brown colour.
- Electrical conductivity is high.
Question 4.
Compare the acid strength of the following compounds
- Nitro methane
- Nitro ethane
- 2 – nitro propane.
Answer:
1. The α – H atom of 10 and 20 nitroalkanes show acidic character because of the electron withdrawing effect of NO2 group.
2. Nitroalkanes dissolve in NaOH solution to form a salt.
3. When the number of alkyl group attached to a carbon increases, acidity decreases. due to +1 effect of alkyl groups.
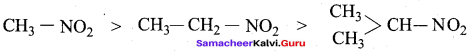
Question 5.
Nitro benzene cannot be prepared from Bromo benzene by direct nitration. Give reason.
Answer:
Nitro benzene cannot be prepared from Bromo benzene because the bromine directly attached to the benzene ring cannot be cleaved easily.
Question 6.
How would you convert Acetaldoxime into Nitroethane?
Answer:
Oxidation of acetaldoxime and with trifluoro peroxy acetic acid gives nitroethane.
![]()
Question 7.
How is nitrobenzene from benzene?
Answer:
When benzene is heated with a nitrating mixture (Con.HNO3 + Con.H2SO4), at 330 K, electrophilic substitution takes place to form nitro benzene.
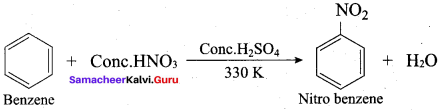
Question 8.
How will you prepare p – dinitrobenzene from p – nitroanlline?
Answer:
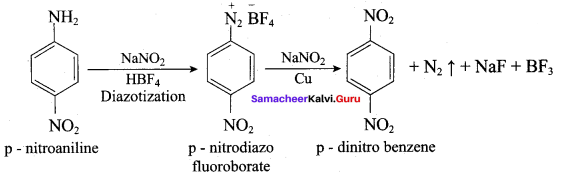
Question 9.
How is amino group can be directly converted into nitro group? Explain with an example.
Answer:
Amino group can be directly converted into nitro group using caro’s acid (H2SO5) (or) persuiphuric acid (H2S2O8) (or) peroxytrifluro acetic acid (F3C.COOOH) as oxidising agent.
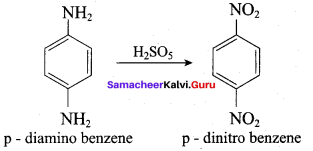
Question 10.
Explain the action of tin and hydrochloric acid with ethyl nitrite.
Answer:
Ethyl nitrite on reduction with Sn / HCI gives ethanol.
![]()
Question 11.
Explain about the acid (or) basic hydrolysis of ethyl nitrite.
Answer:
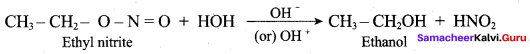
Question 12.
What is Chioropicrin? How is ¡t prepared? Give its uses.
Answer:
CCI3NO2 is Chioropicrin. It is prepared from nitro methane with Cl2 in the presence of NaOH. The a – H atom of nitroalkanes are successively replaced by halogen atoms. It is used as an insecticide.
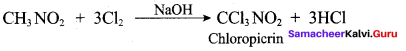
Question 13.
Explain – Nef carbonly synthesis.
Answer:

Question 14.
What happens when nitrobenzene Is treated with Ni (or) Pt (or) LiAIH4?
Answer:
Nitrobenzene undergoes reduction with Ni (or) Pt (or) LiAlH4 to give aniline.
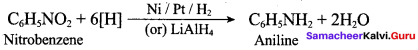
Question 15.
Write a note about structure of Amines.
Answer:
1. Nitrogen atom of amines is trivalent and carries a lone pair of electron and sp3 hybridised, out of the four sp3 hybridised orbitais of nitrogen, three sp3 orbitais overlap with orbitais of hydrogen (or) alkyl groups of carbon, the fourth sp3 orbital contains a lone pair of electron. Hence, amines posses pyramidal geometry.
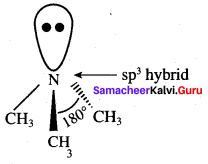
2. Due to presence of lone pair of electron C – N – H (or) C – N – Cbond angle is less than the normal tetrahedral bond angle 109.5v. For example, the C – N – C bond angle of trimethylamine is 108°. This is due to the repulsion between the bulky methyl groups.
Question 16.
How would you convert Nitroethane to ethanamine?
Answer:
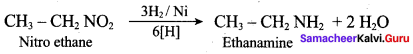
Question 17.
Explain the action of Pt (or) Sn/HCI with nitrobenzene.
Answer:
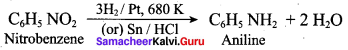
Question 18.
Explain mendlus reaction.
Answer:
Reduction of alkyl or aryl cyanides with Na / C2H5OH is used as a reducing agent is called mendius reaction.
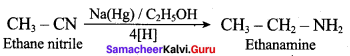
Question 19.
Explain the action of sodium amalgum and ethanol with Methyl isocyanide.
Answer:
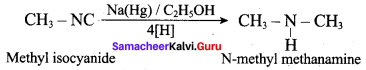
Question 20.
What happens when sodium azide is treated with methyl bromide?
Answer:
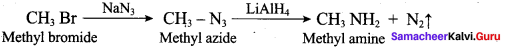
Quesion 21.
How would you convert chiorobenzene to aniline?
Answer:
When chiorobenzene is heated with alcoholic ammonia, aniline is obtained.
![]()
Question 22.
Explain Sabatier – MalIhe method.
Answer:
when vapour of an alcohol and ammonia are passed over alumina, W2O5 (or) silica at 400°C, all types of amines are formed. This method is called Sabatier – Mailhe method.
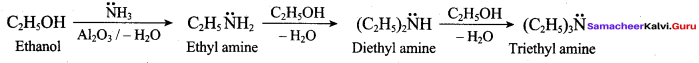
Question 23.
Convert phenol into aniline.
Answer:
Phenol reacts with ammonia at 300°C in the presence of anhydrous ZnCl2 to give aniline.
Question 24.
Compare the boiling points of 10, 2° and 3° amInes.
Answer:
1. The boiling point of various amines follows the order,
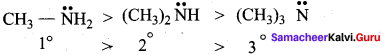
2. Due to the polar nature of primary and secondary amines, can form intermolecular hydrogen bonds using their lone pair of electron on nitrogen atom. However, tertiary amines do not form intermolecular hydrogen bond and they have lower boiling point than 1° and 2° amines.
Question 25.
Aniline is basic in nature. Justify this statement.
Answer:
The lone pair of electrons on nitrogen atom in aniline makes it base. Aniline reacts with mineral acids to form salt.

Question 26.
Alkyl amines are stronger bases than ammonia. Justify this statement.
Answer:
When a + I group like and alkyl group is attached to the nitrogen increase the electron density on nitrogen which makes the electron pair readily available for protonation. Hence alkyl amines are stronger than ammonia.
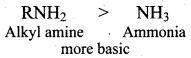
Question 27.
Explain the action of acretyl chloride with ethyl amine?
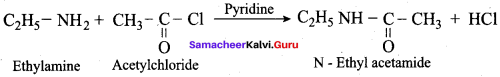
Question 28.
What happens when ethylamine reacts with nitrous acid?
Answer:
Ethylamine reacts with nitrous acid to give ethyl diazonium chloride, which is unstable and it is converted to ethanol by liberating N2.
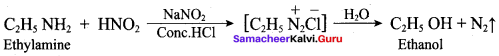
Question 29.
Explain the action of nitrous acid with N – methyl aniline.
Answer:
N – methyl aniline react with nitrous acid to give N – nitroso amine as yellow oily liquid which is insoluble in water.
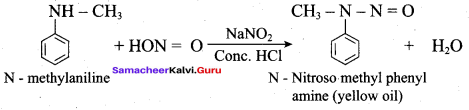
Question 30.
Explain the action of nitrous acid with trimethyl amine.
Answer:
Aliphatic tertiary amine reacts with nitrous acid to form trialkyl ammonium nitrite salt, which is soluble in water.
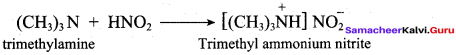
Question 31.
What happens when nitrous acid is treated iith N, N – dimethyl aniline?
Answer:
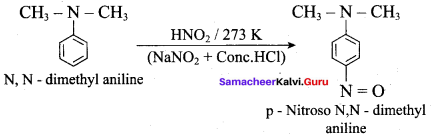
Question 32.
Explain Hoffmann mustard oil reaction. (or) Explain the action of CS2 with aniline.
Answer:
When aniline is treated with CS2, or heated together, S – diphenylthio urea is formed, which on boiling with strong HCI , phenyl isothiocyanate (phenyl mustard oil), is formed.
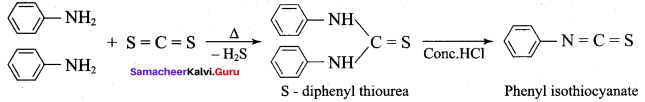
The above reaction is known as Hoffmann mustard oil reaction.
Question 33.
Explain the action of Br2water with aniline.
Answer:
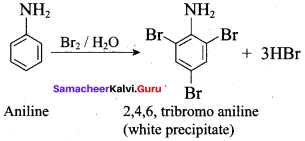
Question 34.
How would you prepare p – bromo aniline from aniline?
Answer:
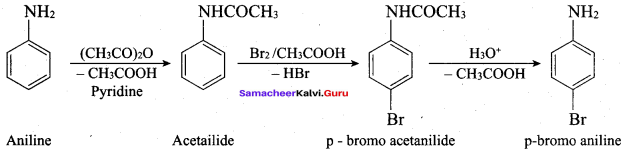
Question 35.
How would you prepare p – nitro from aniline?
Answer:
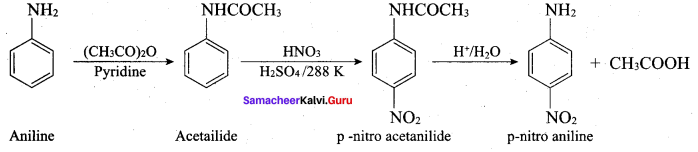
Question 36.
Explain the action of hypophosphrous acid with Benzene diazonium chloride. (or) Explain the action of ethanol with benzene diazonium chloride.
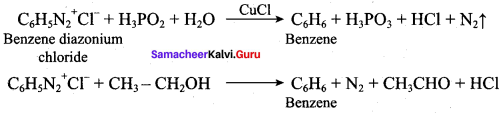
Question 37.
Explain Gattermann reaction.
Answer:
Gattermann reaction.
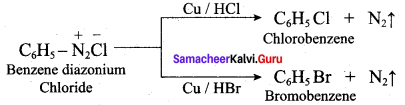
Question 38.
How would you get iodo benzene form benzene diazonium chloride.
Answer:
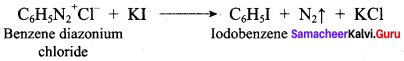
Question 39.
Explain Baltz – schiemann reaction.
Answer:
When benzene diazonium chloride is treated with fluoroboric acid, benezene diazonium tetra fluoroborate is precipitated which on heating decomposes to give fluorobenzene.
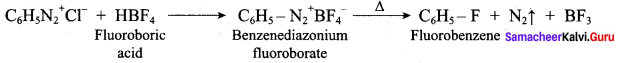
Question 40.
Convert Benzene diazonium chloride into phenol.
Answer:
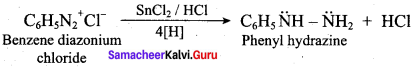
Question 41.
StartIng form Benzene diazonium chloride, how will you get Nitrobenzene?
Answer:
When benzene diazonium chloride is treated with fluoroboric acid we get diazonium fluoroborate which on treated with sodium nitrite solution in the presence of copper, nitrobenzene is obtained.
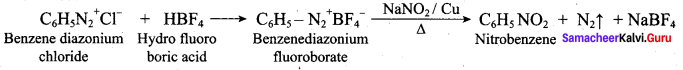
Question 42.
Convert benzene diazonium chloride to benzoic acid
Answer:
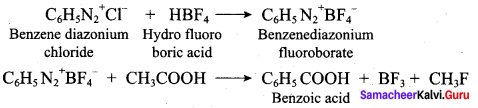
Question 43.
Explain the action of SnCl2 and HCl with benzene diazonium chloride.
Answer:
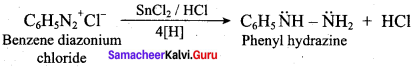
Question 44.
Starting from benzene diazonium chloride, how would you get bright organge azo dye?
Answer:

Question 45.
Write the structural formula and TUPAC name of the following compounds.
- Isobutyl nitrite
- Benzo nitrile
Answer:
1. Isobutyl nitrite
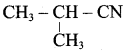
2 – methyll propane nitrile
2. Benzo nitrile C6H5CN Benzene carbo nitrile
Question 46.
Draw the structural formula of
- 3 – cyano butanoic acid
- 2 – bromo – 3 – chloro – 3 – methyl pentane nitrile.
Answer:
1. 3 – cyano butanoic acid
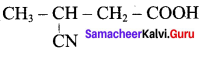
2. 2 – bromo – 3 – chloro
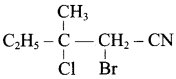
– 3 – methyl pentane nitrile
Question 47.
How will you get propane nitrile from ethyl bromide?
Answer:
![]()
Question 48.
Starting from methyl magnesium bromide, how would you obtain ethane nitrile?
Answer:
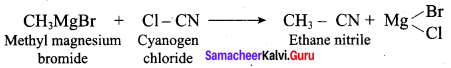
Question 49.
Explain thrope nitrite condensation.
Answer:
Self condensation of two molecules of alkyl nitrile in the presence of sodium to form iminonitrile is called Thrope nitnie condensation.
Question 50.
Explain Levine and hauser acetylation.
Answer:
The nitrites containing α – hydrogen also undergo condensation with esters in the presence of sodamide in ether to form ketoriitriles. This reaction is known as Levine and hauser acetylation.
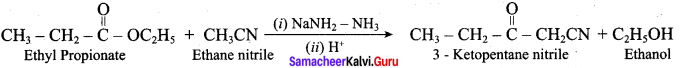
Question 51.
How would you prepare the following compounds by carbylamines reaction.
- Methyl isocyanide
- Phenyl isocyanide
Answer:
1. ![]()
2. ![]()
Question 52.
Complete the following reactions.
Answer:
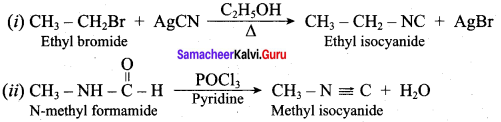
Question 53.
How is methyl isocyanide changed to methyl cyanide?
Answer:
![]()
Question 54.
What are the uses of nitrobenzene?
Answer:
- Nitro benzene is used to produce lubricating oils in motors and machinery.
- It is used in the manufacture of dyes, drugs, pesticides, synthelic rubber, aniline and explosives like TNT, TNB.
Question 55.
Primary amines have higher boiling points than tertiary amines why?
Answer:
Due to the presence of two H – atom on N – atoms of primary amines they undergo extensive intermolecular H – bonding while tertiary amines due to the absence of a H – atom on the N – atom do not undergo H – bonding. As a result primary amines have higher boiling points than tertiary amines.
Question 56.
How is m – nitroaniline obtained from nitrobenzene?
Answer:
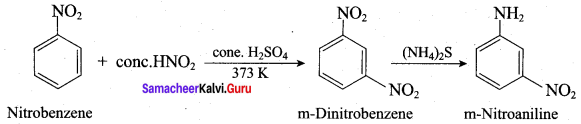
Question 57.
How is aniline obtained from benzoic acid?
Answer:
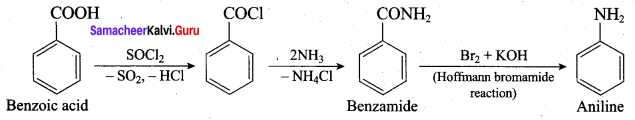
Question 58.
How will you convert benzene into aniline?
Answer:
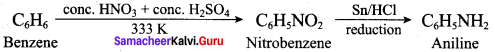
Question 59.
How will you distinguish between.
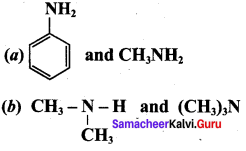
Answer:
1. By reacting with NaNO2 and HCl at the temperature of around 0 to 5°C, Aniline will from diazonium salt. CH3NH2 will form methanol and bubbles of N2 gas will come out of the solution.
2. By Hinsberg’s reagent, C6H5SO2CI(CH3)3N will not react. (CH3)2NH will form a product which is insoluble in alkali.
Question 60.
Account for any two of the following.
- Amines are basic substances while amides are neutral.
- Aromatic amines are weaker bases than aliphatic amines.
Answer:
1. In amines alkyl group is an electron releasing group which increases the electron density on nitrogen thus making them basic whereas in amides
![]()
group is electron withdrawing, therefore they are neutral.
2. It is because aryl group is an electron withdrawing group which decreases electron density on nitrogen atom, making them less basic whereas alkyl group is electron releasing which makes alkylamines more basic.
Question 61.
- Assign a reason for the following statements – Alkylamines are stronger bases than arylamines.
- How would you convert methylamine into ethylamine?
Answer:
1. It is because in arylamines the – NH2 group is attached directly to the benzene ring. It results in the unshared electron pair on nitrogen atom to be in conjugation with the benzene ring and thus making it less available for protonation whereas alkyl group are electron releasing group.
2. 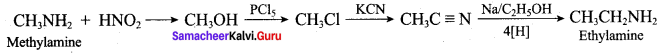
Question 62.
- How will you convert an alkyl halide to a primary amine whose molecule has one carbon atom more than the used alkyl halide molecule?
- Why are amines more basic than the comparable alcohols.
Answer:
1.
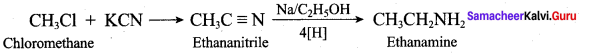
2. Due less electronegativity of oxygen atom as compared to nitrogen, amines are more basic than alcohols.
Question 63.
Aniline gets coloured on standing in air for a long time. Why?
Answer:
Due to strong electron – donating effect (+ R effect) of NH2 group, the electron density on the benzene ring increases. As a result, aniline is easily oxidised on standing in air for a long time to form coloured products.
Question 64.
CH3CONH2 is a weaker base than CH3CH2NH2. Why?
Answer:
Due to resonance, the lone pair of electrons on the nitrogen atom in CH3CONH2 is delocalised over the keto group. There is no such effect ip CH3CH2NH2. Due to reduction in electron density on N – atom of CH3CONH2, it is a weaker base than CH3CH2NH2.
Question 65.
Write chemical equation for the following conversions:
- CH3CH2 – Cl into CH3CH2CH2 – NH2
- C6H5 – CH2 – Cl into C6H5CH2CH2NH2
Answer:
1. 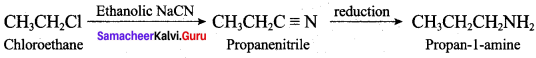
2. 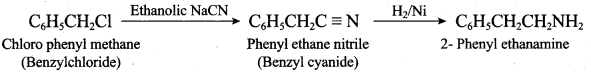
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds 3 Marks Questions and Answers
Question 1.
Write about the classification of organic nitro compounds.
Answer:
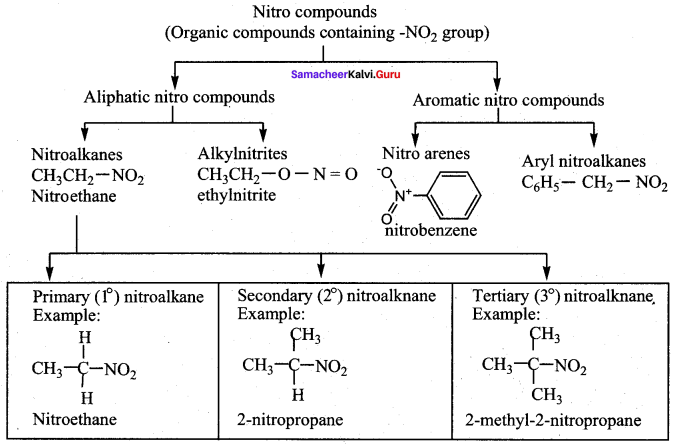
2. Draw the structural formula of the following compounds.
- 2 – methyl – 1 – nltropropane
- 2, 2 – dimethyl – 1 – nitropropane
- Nitrobenzene
Answer:
1. 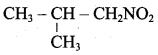 2 – methyl – 1 – nitropropane
2 – methyl – 1 – nitropropane
2. 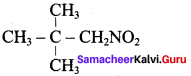 2, 2 – dimethyl – 1- nitropropane
2, 2 – dimethyl – 1- nitropropane
3. 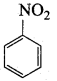 Nitrobenzene
Nitrobenzene
Question 3.
Draw the structural formula of the following compounds.
- 2 – nitro – 1 – methyl benzene
- 1, 3, 5 – trinitrobenzene
- 2 – phenyl – 1 – nitroethane
Answer:
1.  2 – nitro – 1 – methyl benzene
2 – nitro – 1 – methyl benzene
2. 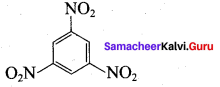 1, 3, 5 – trinitrobenzene
1, 3, 5 – trinitrobenzene
3.  2 – phenyl – 1 – nitroethane
2 – phenyl – 1 – nitroethane
Question 4.
Write the possible isomers for the formula C4H9NO2.
Answer:
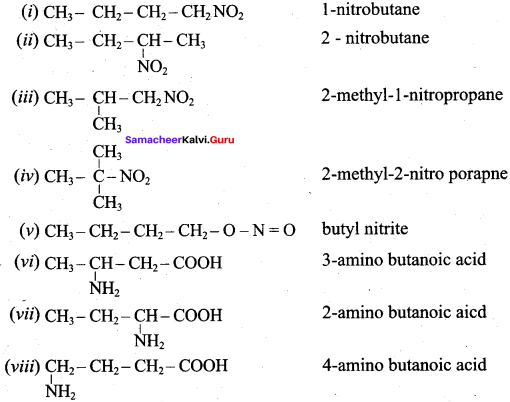
Question 5.
How would you prepare nitro ethane from the following compounds?
- CH3 – CH2Br
- CH3 – CH3
Answer:
1. ![]()
2. 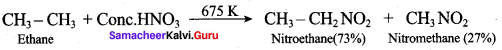
Question 6.
Mention any two methods of preparation of nitro methane.
Answer:
1. ![]()
2. ![]()
Question 7.
Explain the
- acid medium reduction
- neutral medium reduction of Nitromethane.
Answer:
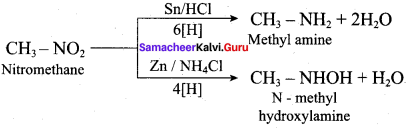
Question 8.
What happens when hydrochloric acid is treated with
- Nitro ethane
- 2 – nitropropane
- 2 – methyl – 2 – nitro propane
(or)
How would you distinguish 1°, 2°, 3° nitro compounds?
Answer:
1. ![]()
2. 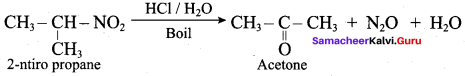
3. 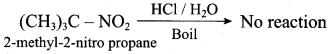
Question 9.
Explain the following reactions using nitro benzene.
- Chlorination
- Nitration
- Suiphonation
Answer:
1. 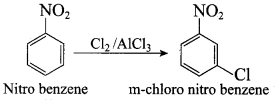
2. 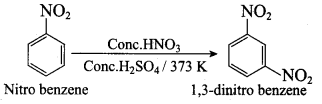
3. 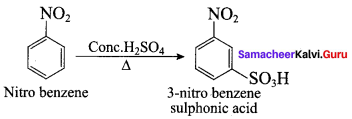
Question 10.
Give the structural formula and IUPAC name of the following compounds.
- Isopropvl amine
- Allyl amine
- Hexamethyl diamine
Answer:
1. Isopropvl amine Propan – 2 – amine
Propan – 2 – amine
2. Allyl amine ![]() Prop – 2 – en – 1 – amine
Prop – 2 – en – 1 – amine
3. Hexamethyl diamine ![]() Hexane 1, 6 – diamine
Hexane 1, 6 – diamine
Question 11.
Draw the structural formula and write the IUPAC name of
- Methyl isopropyl amine
- Diethyl butyl amine
- Ethyl methyl ispropylamine
Answer:
1. Methyl isopropyl amine:
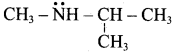
N – methyl propan – 1 – amine
2. Diethyl butyl amine:
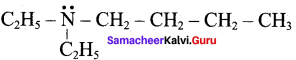
N, N – diethyl butan – 1 – amine
3. Ethyl methyl ispropylamine:
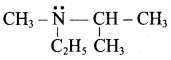
N – ethyl – N – methyl propan – 2 – amine
Question 12.
Draw the structural formula and write the IUPAC name of …………..
- N, N – dimethyl aniline
- Benzyl amine
- N – methyl benzylamine
Answer:
1. N, N – dimethyl aniline
2. Benzyl amine
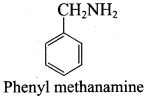
3. N – methyl benzylamine
Question 13.
Explain the alkylation reaction of methylamine with equation.
Answer:
Alkylation:
Primary amines reacts with alkyl halides to give successively 2° and 3° amines and quaternary ammonium salts.
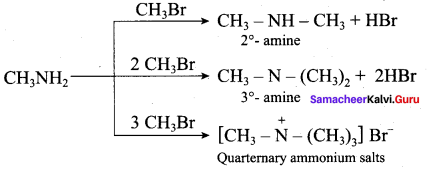
Question 14.
Explain about the suiphonation reaction of aniline.
Answer:
Aniline reacts with Conc. H2SO4 to form anilinium hydrogen sulphate which on heating with H2SO4 at 453 – 473K gives p – aminobenzene suiphonic acid, commonly known as suiphanilic acid, as the major product.
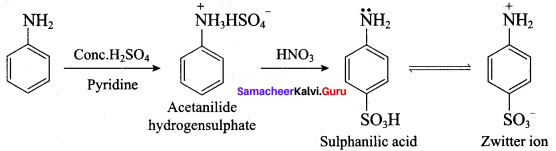
Question 15.
Explain Sandmeyer reaction with example.
Answer:
On mixing freshly prepared solution of benzene diazonium chloride with cuprous halides, aryl halides are formed. This reaction is called Sandmeyer reaction.
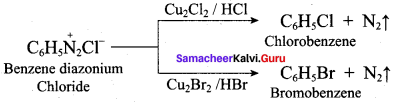
Question 16.
Write the structural formula and IUPAC name of the following compounds.
- Methyl cyanide
- Propionitrile
- Butyro nitrile
Answer:
- CH3CN – Ethane nitrile
- CH3 – CH2CN – Propane nitrile
- CH3 – CH2 – CH2CN – Butane nitnie.
Question 17.
How would you produce Ethane nitrite form the following compounds?
- Acetamide
- Ammonium acetate
- Acetaldoxime
Answer:
1. 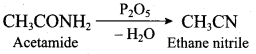
2. 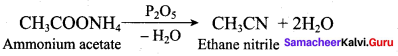
3. 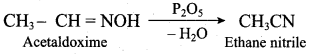
Question 18.
Explain the action of following reagent with ethane nitrile.
- Dilute mineral acid
- Ni / H2
Answer:
1. 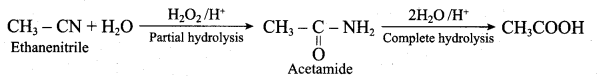
2. 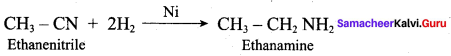
Question 19.
What happens when methyl isocyanide reacts with the following reagents?
- Mineral acid
- Na + C2H5OH
Answer:
1. 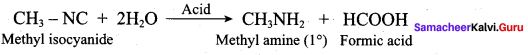
2. 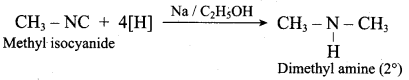
Question 20.
Explain the addition reactions of alkyl Isocyanide with
- halogen
- sulphur
- ozone
Answer:
1. 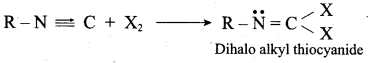
2. ![]()
3. ![]()
Question 21.
What are the uses of aliphatic nitro compounds.
Answer:
- Nitromethane is used as a fuel for cars.
- Chloropicrrn (CCI3NO2) is used as an insecticide
- Nitroethane is used as a fuel additive and precursor to explosive and they are good solvents for polymers, cellulose ester, synthetic rubber and dyes etc,
Question 22.
Explain about the structure and uses of Mitomycin.
Answer:
- Mitomycin C, and anticancer agent used to treat stomach and colon cancer, contains an aziridine ring.
- The aziridine functional group participates in the drug’s degradation by DNA, resulting in the death of cancerous cells.
Question 23
Complete the following reactions.
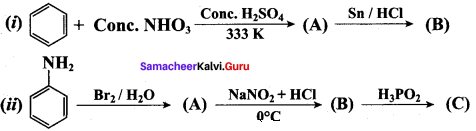
Answer:
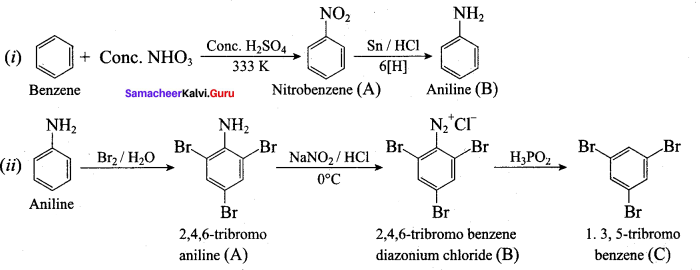
Question 24.
Convert Methanamine into Ethanamine.
Answer:
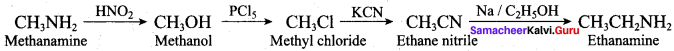
Question 25.
Convert Ethanamic into Methanamine.
Answer:
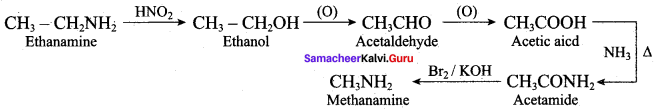
Question 26.
How would you obtain Benzoic acid from aniline?
Answer:
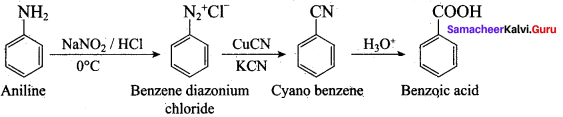
Question 27.
Complete the following reactions.
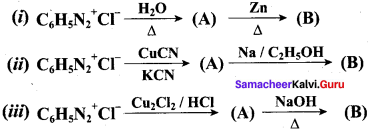
Answer:
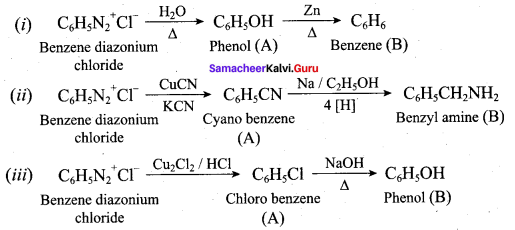
Question 28.
Write the reactions of
- aromatic and
- aliphatic primary amines with nitrous acid.
Answer:
Aromatic primary amines react with HNO2 at 273 – 278 K to form aromatic diazonium salts.

Aliphatic primary amines also react with HNO2 at 273 – 278 K to form aliphatic diazonium salts. But these are unstable even at low temperature and thus decomposes readily to form a mixture of compounds consisting of alkyl chlorides, alkenes and alcohols, out of which alcohols generally predominates.
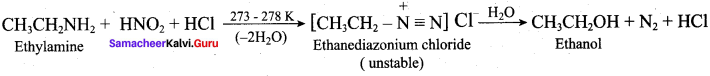
Question 29
Given plausible explanation for each of the following.
- Why are amines less acidic than alcohols of comparable molecular masses?
- Why do primary amines have higher boiling point than tertiary amines?
- Why are aliphatic amines stronger bases than aromatic amines?
Answer:
1. It is because C2H5O° is more stable than C2H5NH° because oxygen is more electronegative than nitrogen.
2. Due to the presence of two H – atoms on N – atom of primary amines, they undergo extensive intennolecular H – bonding while tertiary amines due to the absence of a H – atoms on the N – atom do not undergo H – bonding. As a result, primary amines have higher boiling point than tertiary amines of comparable molecular masses.
3. It is because there is electron withdrawing, C6H5 group in aromatic amines which makes them less basic than aliphatic amines in which alkyl group is electron releasing.
Question 30.
Account for the following.
- Primary amines (R – NH2)have higher boiling point than tertiary amines (R3N).
- Aniline does not undergo Friedel – Crafts reaction.
- (CH3)2NH Is more basic than (CH3)3N in an aqueous solution.
Answer:
1. Due to maximum intermolecular hydrogen bonding in primary amines (due to presence of more number of H – atoms), primary amines have higher boiling point in comparison to tertiary amines.
2. Aniline does not undergo Friedel-Crafts reaction due to acid-base reaction. Aniline and a Lewis Acid / Protic Acid, which is used in Friedel-crafts reaction.
3. In (CH3)3N there is maximum steric hindrance and least solvation but in (CH3)2NH the solvation is more group; di-methyl amine is still a stronger base than trimethyl amine.
Question 31.
Write the structures of A,B and C in the following reactions.
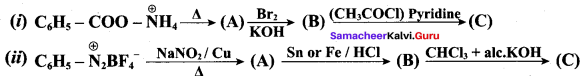
Answer:
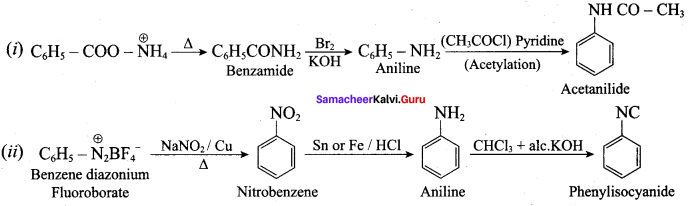
Question 32.
Predict, giving reasons the order of basicity of the following compounds.
- gaseous phase
- In aqueous solution (CH3)3N, (CH3)2NH, CH3NH2, NH3
Answer:
1. In gaseous phase, basic character of amines increases with the increase in the number of electron the releasing alkyl groups due to + I effect so the trend of basic character is 3° > 2° > 1° > NH3. Therefore, (CH3)3N > (CH3)3NH > CH3NH2 > NH3.
2. In aqueous phase, solvation of ammonium cation occurs by warer molecules, greater the size of ion, lesser will be the solvation and lesser will be the stability of ion. So on combining + I effect and solvation effect, in aqueous phase trend changes to 2° > 1° > 3°, (CH3)2NH > CH3NH2 > (CH3)3N > NH3.
Question 33.
Identify A and B in the following reactions.
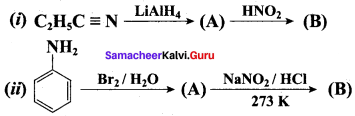
Answer:
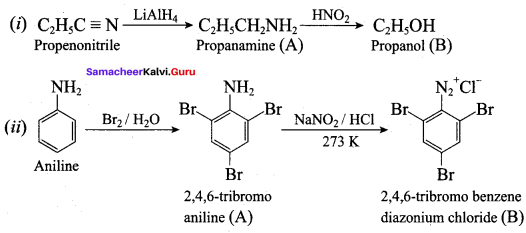
Question 34.
What happens when. (Write reactions only)
- Nitroethane is treated with LiAlH4.
- Diazonium chloride reacts with phenol in basic medium.
Answer:
1. 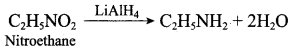
2. 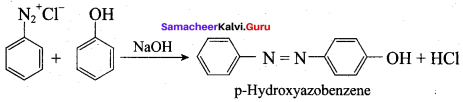
Question 35.
How would you achieve the following conversions.
Answer:
(i) Nitrobenzene to aniline
(ii) An alkyl halide to a quarternary ammonium salt.
(iii) Aniline to benzonitrile.
Answer:
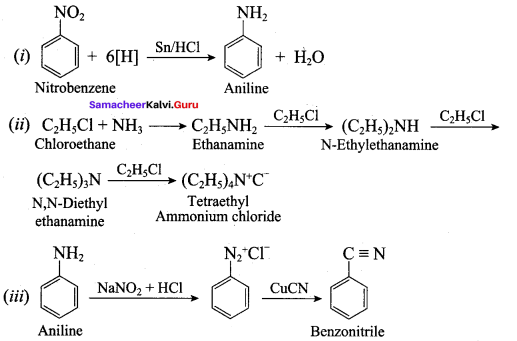
Question 36.
What happens when (write reactions only).
(i) Nitropropane is treated with LiAlH4.
(ii) Ethyl lsocyañide undergoes hydrolysis.
(iii) Benzene diazonium chloride reacts with phenol i basic medium.
Answer:
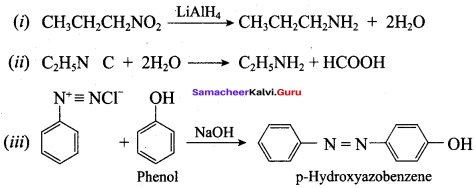
Question 37.
Identify A and B in the following reactions.
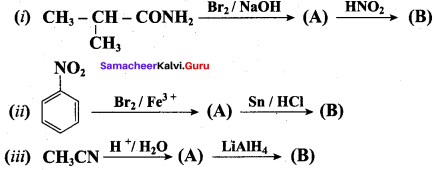
Answer:
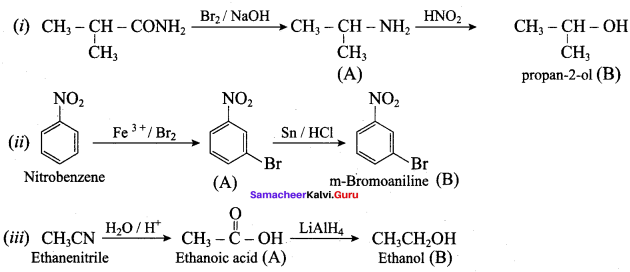
Question 38.
Identify A and B in the following reactions
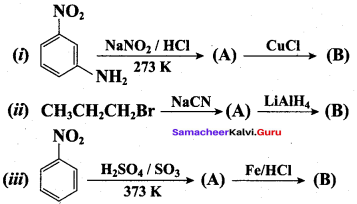
Answer:
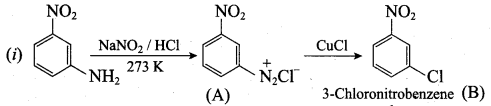
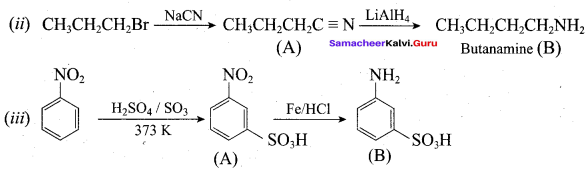
Question 39.
A compound ‘x’ having molecular formula C3H7NO reacts with Br2 in the presence of KOH to give another compound ‘y’. The compound ‘y’ reacts with HNO2 to form ethanol. and N2 gas. Identify the compounds x andy and write the reactions involved.
Answer:
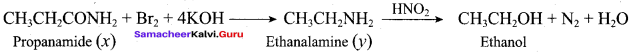
Question 40.
An organic compound ‘A’ having molecular formula C3H5N on hydrolysis gave a nother compound ‘B’ The compound ‘B’ on treatment with HNO2 gave ethyl alcohol. ‘B’ on warming with CHCI3 and alcoholic caustic potash gave an offensive smelling substance ‘C’. Identify ‘A’, ‘B’ and ‘C’.
Answer:
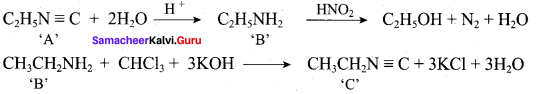
Samacheer Kalvi 12th Chemistry 13 Organic Nitrogen Compounds 5 Mark Questions and Answers
Question 1.
Explain the various reduction reactions of nitrobenzene.
Answer:
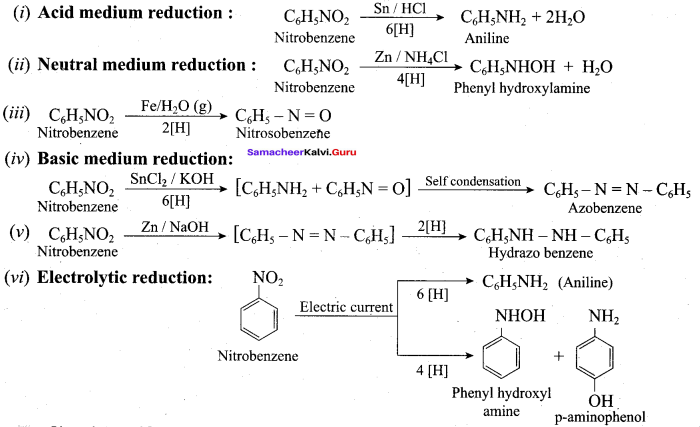
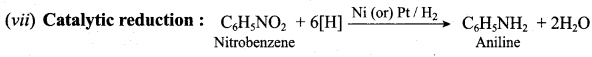
Question 2.
What happens when nitrous acid react with
(i) Ethyl amine
(ii) Aniline
(iii) N – methyl aniline
(iv) Trimethyl amine
(v) N, N – dimethyl aniline
Answer:
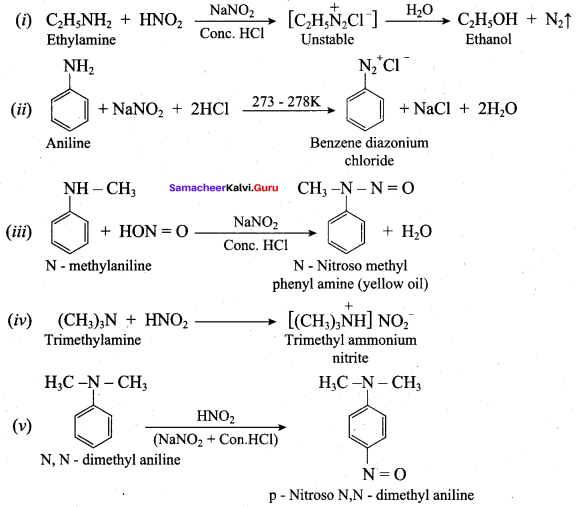
Question 3.
Starting from Benzene diazonium chloride, how would you prepare
(i) Benzene
(ii) Phenol
(iii) Nitro benzene
(iv) Benzolc acid
(v) Fluorobenzene
Answer:
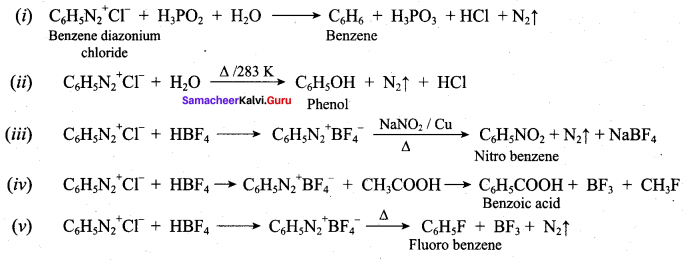
Question 4.
Starting from Benzene diazonium chloride, how would you prepare
(i) Biphenyl
(ii) Phenyl hydrazine
(iii) p – hydroxy azo benzene
(iv) p – amino azo benzene
(v) Chioro benzene
Answer:
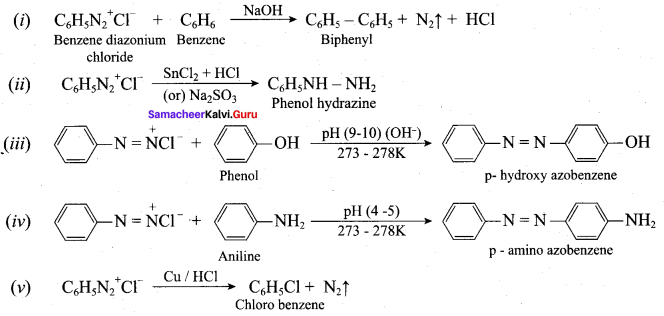
Question 5.
Convert aniline into the following compounds.
(i) N – phenyl benzamide
(ii) Phenyl isothiocyanate
(iii) 2, 4, 6 – tribromo aniline
(iv) Sulphanilinic acid
(v) Phenyl isocyanide
Answer:
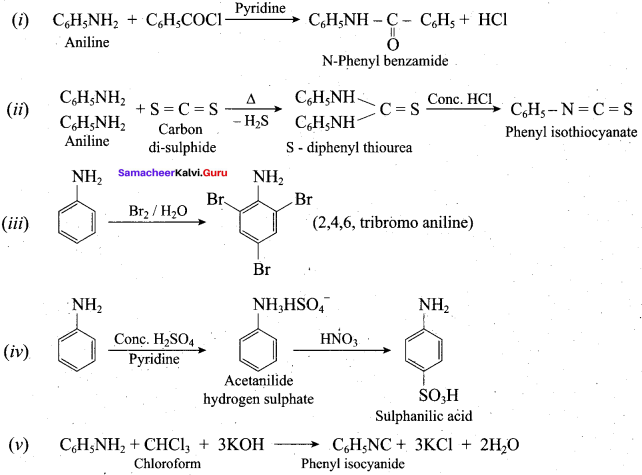
Question 6.
An organic compound (A) of molecular formula C6H7N on reaction with sodium nitrite and hydrochloric acid at 0°C gives (B) of formula C6H5N2Cl. (B) on treatment with cuprous cyanide give (C) of formula C7H5N. (C) on reaction with sodium and ethanol gives (D) of formula C7H9N. (D) on reaction with nitrous acid gives (E) of molecular formula C7H8O. Identify A, B, C, D and E and explain the reactions involved.
Answer:
1. (A) is identified as Aniline from the molecular formula.
2. Aniline on reaction with sodium nitrite and hydrochloric acid at 0°C, diazotisation take place and the product (B) formed is benzene diazonium chloride.
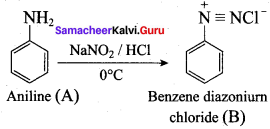
3. Benzene diazonium chloride on treatment with CuCN produces (C) as cyano benzene.
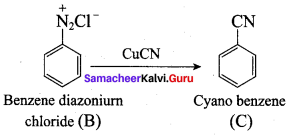
4. Cyano benzene on reaction with Na and C2H5OH undergoes reduction reaction to give (D) as benzvlamine.
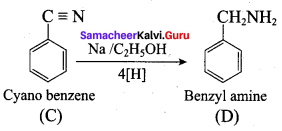
5. Benzylamine on treatment with nitrous acid gives benzyl alcohol as (E).
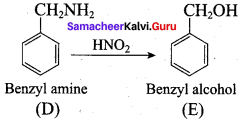
Question 7.
Complete the following reactions and identify the A, B and C in these reaction.
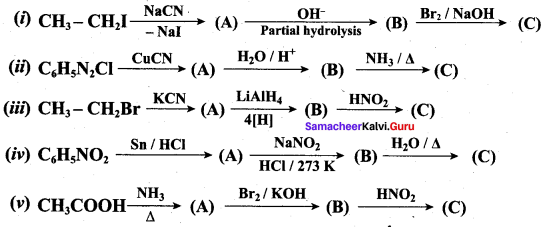
Answer:
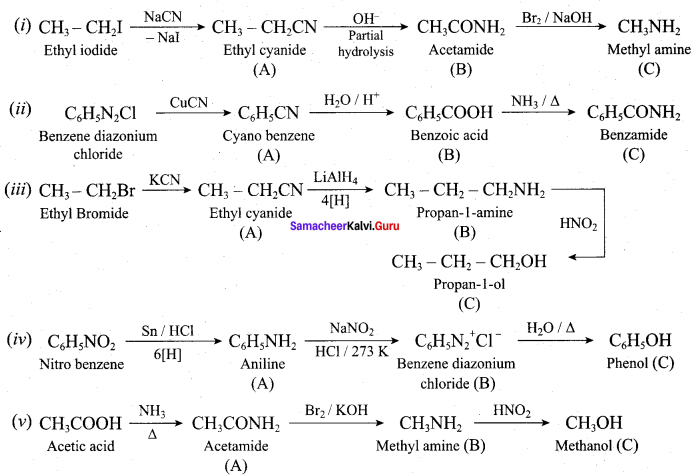
Question 8.
An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compound A, B and C.
Answer:
Step – 1: To find out the structure of compounds ‘B’ and ‘C’.
1. Since compound ‘C’ with molecular formula C6H7N is formed from compound ‘B’ on treatment with Br2 + KOH (i.e., Hoffmatm bromamide reaction). Therefore, compound ‘B’ must be an amide and ‘C’ must be an amine. The only amine having the molecular formula C6H7N is C6H5NH2 (i.e., aniline or benzenamine).
2. Since ‘C’ is aniline, therefore, the amide from which it is formed must be benzamide (C6H5CONH2). Thus, compound ‘B’ is benzamide. The chemical equation showing the conversion of ‘B’ to ‘C’ is

Step – 2:
To find out the structure of compound ‘A’. Since compound ‘B’ is formed from compound ‘A’ by treatment with aqueous ammonia and heating. Therefore, compound ‘A’ must be benzoic acid or benzenecarboxylic acid.
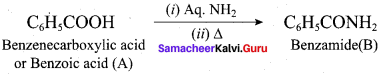
Question 9.
An aromatic compound ‘A’ of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A,B,C, D and E in the following reactions.
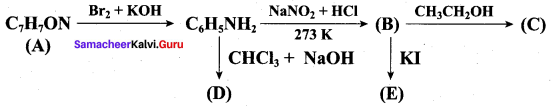
Answer:
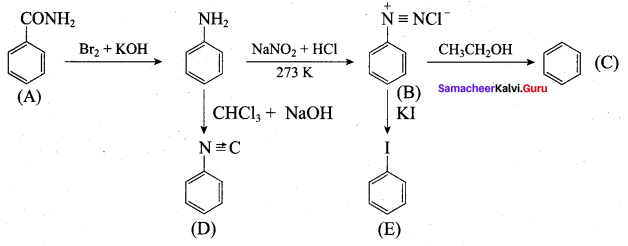
Question 10.
Write reactions and conditions required for the following conversions.
(i) Aniline to benzene
(ii) Methylamine to methylcyanide
(iii) Propanenitrile to ethylamine
(iv) m – Bromoaniline to m – bromophenol
(v) Nitrobenzene to 2, 4, 6 – tribromoaniline.
Answer:
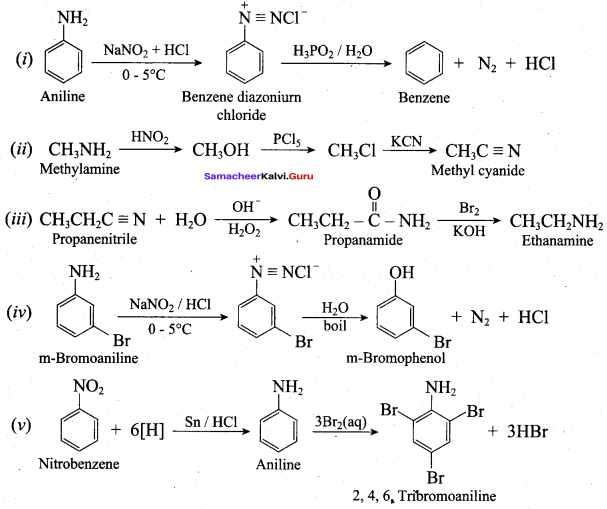
Question 11.
A compound ‘A’ of molecular formula C3H7O2N on reaction with Fe and cone. HCI gives a compound ‘B’ of molecular formula C3H9N. Compound ‘B’ on treatment with NaNO2 and HCI gives another compound ‘C’ of molecular formula C3H8O. The compound ‘C’ has molecular formula, C3H8O. The compound ‘C’ gives effervescence with Na. On oxidation with CrO3, the compound ‘C’ gives a saturated aldehyde containing three carbon atoms. Deduce the structures of A, B and C and write the equations for the reaction involved.
Answer:
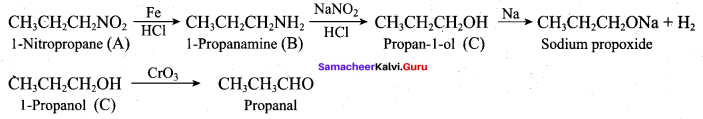
Question 12.
Identify compounds A, B and C in the following reactions.
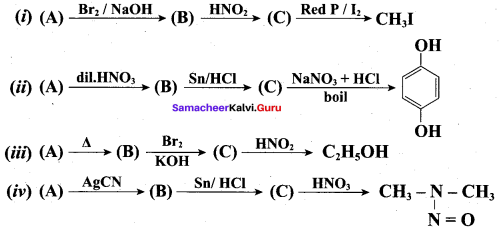
Answer:
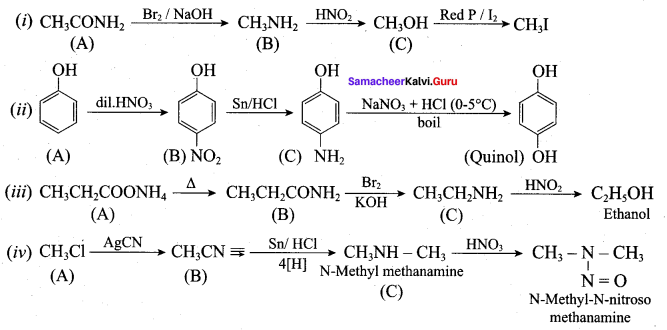
Question 13.
A aromatic hydrocarbon (A) of molecular formula C6H6 reacts with Conc.HNO3 and Conc. H2SO4 gives (B) of formula C6H5O2N. (B) on reaction with Sn/HCI gives (C) of formula C6H7N which answers carbylamine reaction. (C) on treatment with chloroform and alkali gives (D) of formula C7H5N. Identify A, B, C, D and explain the reactions involved.
Answer:
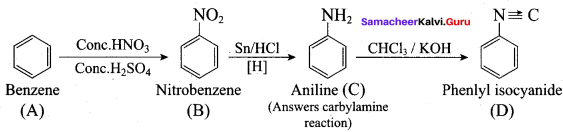
Question 14.
Convert the following.
(i) Nitro benzene → Benzene
(ii) Benzene → Benzoic acid
Answer:
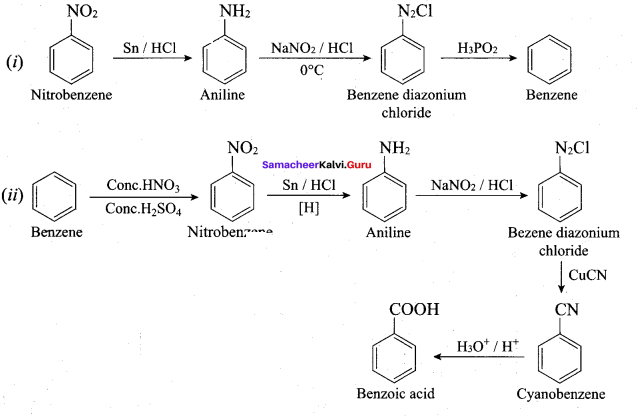
Common Errors
- TUPAC name of nitro compounds.
- If the formula ends as – NO2, the students always think of nitro only.
- Reducing agents may be different for different medium.
- Basic character of amines is always difficult to remember.
- Cyanide and isocyanide formula may get confusing.
Rectifications
1. Both common name and IUPAC names are same for aliphatic and aromatic nitro compounds.
2. – NO2 may be

3.
- Acid medium reducing agent: Sn / HCI
- Neutral medium reducing agent: Zn + NH4CI
- Basic medium reducing agent: Zn / NaOH
- Catalytic reducing agent: Ni, Pt, LiAlH4
4. 2° amine > 3° amine > 1° amine > NH3

Alkyl group is connected to ![]() C – Cyanide. If alkyl group connected to
C – Cyanide. If alkyl group connected to ![]() Isocyandie.
Isocyandie.
We hope the Class 12th Samacheer Kalvi Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Book Solutions Answers Guide Solutions provided here help the students to get the best knowledge. Also, students can use our Samacheer Kalvi Class 12th Chemistry Solutions Chapter 13 Organic Nitrogen Compounds Questions and Answers for better practice. You can contact us at any moment by giving comments in the comment section. Keep checking updates by bookmarking our website.