Tamilnadu State Board Solutions for Class 12th Chemistry Solutions Chapter 14 Biomolecules Questions and Answers will help you to improve the complete subject knowledge. Every student must look at the single concept included in Samacheer Kalvi 12th Chemistry Solutions Chapter 14 Biomolecules Book Solutions Answers Guide Solutions PDF. All the concepts are explained clearly with examples and pictures.
Students can easily avoid the struggle to get the best book for Chemistry Solutions Chapter 14 Biomolecules Book Solutions Answers Guide learning. You have to go through Chapterwise Samacheer Kalvi Class 12th Textbook Solutions for Chemistry Solutions Chapter 14 Biomolecules Book Solutions for better practice.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 14 Biomolecules
Students who wish to have the strong basics of Chemistry Solutions Chapter 14 Biomolecules can use Tamilnadu State Board Class 12th Chemistry Solutions Chapter 14 Biomolecules Questions and Answers Guide pdf. Enhance your knowledge by referring to the Samacheer Kalvi Solutions pdf. We provided a free pdf of Tamilnadu State Board Class 12th Chemistry Solutions Chapter 14 Biomolecules Book Solutions Answers Guide material and textbook for students. Check it out now and start preparing for the exam immediately. Score maximum marks in the exam by referring to Samacheer Kalvi Class 12th Chemistry Solutions Chapter 14 Biomolecules Book Solutions Answers Guide Solutions Pdf.
Samacheer Kalvi 12th Chemistry Chapter 14 Biomolecules Textual Evaluation Solved
Samacheer Kalvi 12th Chemistry Biomolecules Multiple Choice Questions
Question 1.
Which one of the following rotates the plane polarized light towards left?
(a) D(+) Glucose
(b) L(+) Glucose
(c) D(-) Fructose
(d) D(+) Galactose
Answer:
(c) D(-) Fructose
Question 2.
The correct corresponding order of names of four aldoses with configuration given below Respectively is ………………..
(a) L – Erythrose, L – Threose, L – Erythrose, D – Threose
(b) D – Threose, D – Erythrose, L – Thrcose, L – Erythrose
(c) L – Etythrose, L – Threose, D – Erythrose, D – Threose
(d) D – Erythrose, D – Threose, L – Erythrose, L – Threose
Answer:
(d) D – Erythrose, D – Threose, L – Erythrose, L – Threose
Question 3.
Which one given below is a non-reducing sugar?
(a) Glucose
(b) Sucrose
(c) maltose
(d) Lactose
Answer:
(b) Sucrose
Question 4.
Glucose(HCN) Product (hydrolysis) Product (HI + Heat) A, the compound A is ………………..
(a) Heptanoic acid
(b) 2 – lodohexane
(c) Heptane
(d) Heptanol
Answer:
(a) Heptanoic acid
Question 5.
Assertion: A solution of sucrose in water is dextrorotatory. But on hydrolysis in the presence of little hydrochloric acid, it becomes levorotatory.
Reason: Sucrose hydrolysis gives unequal amounts of glucose and fructose. As a result of this change in sign of rotation is observed.
(a) If both accretion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true but reason is not the correct explanation of assertion
(c) If assertion is true but reason is false
(d) if both assertion and reason are false
Answer:
(a) If both accretion and reason are true and reason is the correct explanation of assertion
Qustion 6.
The central dogma of molecular genetics states that the genetic information flows from ……………..
(a) Amino acids Protein DNA
(b) DNA Carbohydrates Proteins
(c) DNA RNA Prnteins
(d) DNA RNA Carbohydrates
Answer:
(c) DNA RNA Proteins
Question 7.
In a protein, various amino acids liked together by …………..
(a) Peptide bond
(b) Dative bond
(c) α – Glycosidic bond
(d) β – Glycosidic bond
Answer:
(a) Peptide bond
Question 8.
Among the following the achiral amino acid is …………..
(a) 2 – ethylalanine
(b) 2 – methyiglycine
(c) 2 – hydroxymethyiscrine
(d) Tryptophan
Answer:
(c) 2 – hydroxymethylserine
Question 9.
The correct statement regarding RNA and DNA respectively is ………………
(a) the sugar component in RNA is an arabinose and the sugar component in DNA is ribose
(b) the sugar component in RNA is 2’ – deoxyribose and the sugar component in DNA is arabinose
(c) the sugar component in RNA is an arabinose and the sugar component in DNA is 2’ – deoxyribose
(d) the sugar component in RNA is ribose and the sugar component in DNA is 2’-deoxyribose
Answer:
(d) the sugar component in RNA is ribose and the sugar component in DNA is 2’ – deoxyribose
Question 10.
In aqueous solution of amino acids mostly exists in ……………….
(a) NH2 – CH(R) – COOH
(b) NH2 – CH(R) – COO
(c) H3N – CH(R) – COOH
(d) H3N+ – CH(R) – COO–
Answer:
(d) H3N+ – CH(R) – COO–
Question 11.
Which one of the following is not produced by body?
(a) DNA
(b) Enzymes
(c) Hormones
(d) Vitamins
Answer:
(d) Vitamins
Question 12.
The number of sp2 and sp3 hybridised carbon in fructose are respectively ……………..
(a) 1 and 4
(b) 4 and 2
(c) 5 and 1
(d) 1 and 5
Answer:
(d) 1 and 5
Question 13.
Vitamin B2 is also known as …………….
(a) Riboflavin
(b) Thiamine
(c) Nicotinamide
(d) Pyridoxine
Answer:
(a) Riboflavin
Question 14.
The pyrimidine bases present in DNA are …………..
(a) Cytosine and Adenine
(b) Cytosine and Guanine
(c) Cytosine and Thiamine
(d) Cytosine and Uracil
Answer:
(c) Cytosine and Thiamine
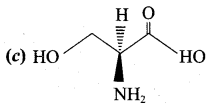
Question 15.
Among the following L – serine is …………..
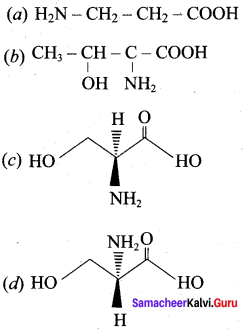
Answer:
Question 16.
The secondary structure of a protein refers to ………………
(a) fixed configuration of the polypeptide backbone
(b) hydrophobic interaction
(c) sequence of a-amino acids
(d) α – helical backbone
Answer:
(d) α – helical backbone
Question 17.
Which of the following vitamins is water soluble?
(a) Vitamin E
(b) Vitamin K
(c) Vitamin A
(d) Vitamin B
Answer:
(b) Vitamin K
Question 18.
Complete hydrolysis of cellulose gives ……………
(a) L – Glucose
(b) D – Fructose
(c) D – Ribose
(d) D – Glucose
Answer:
(d) D – Glucose
Question 19.
Which of the following statement is correct?
(a) Ovalbumin is a simple food reserve in egg-white
(b) Blood proteins thrombin and fibrinogen are involved in blood clotting
(c) Denaturation makes protein more active
(d) Insulin maintains the sugar level of in the human body
Answer:
(c) Denaturation makes protein more active
Question 20.
Glucose is an aldose. Which one of the following reactions is not expected with glucose?
(a) It does not form oxime
(b) It does not react with Grignard reagent
(c) It does not form osazones
(d) It does not reduce tollens reagent
Answer:
(b) It does not react with Grignard reagent
Question 21.
If one strand of the DNA has the sequence ‘ATGCTTGA’, then the sequence of complementary strand would be ……………..
(a) TACGAACT
(b) TCCGAACT
(c) TACGTACT
(d) TACGRAGT
Answer:
(a) TACGAACT
Question 22.
Insulin, a hormone chemically is ………….
(a) Fat
(b) Steroid
(c) Protein
(d) Carbohydrates
Answer:
(c) Protein
Question 23.
a – D (+) Glucose and ß – D (+) glucose are ………………..
(a) Epimers
(b) Anomers
(c) Enantiomers
(d) Conformational isomers
Answer:
(b) Anomers
Question 24.
Which of the following are epimers?
(a) D(+) – Glucose and D(+) – Galactose
(b) D(+) – Glucose and D(+) – Mannose
(c) Neither (a) nor (b)
(d) Both (a) and (b)
Answer:
(d) Both (a) and (b)
Question 25.
Which of the following amino acids are achiral?
(a) Alanine
(b) Leucine
(c) Proline
(d) Glycine
Answer:
(a) Alanine
II. Answer the following questions.
Question 1.
What type of linkages hold together monomers of DNA?
Answer:
- Phospho diester linkages hold together monomers of DNA
- Phosphoric acid forms phospho diester bond between neucleotides
- The sugar – phosphate linkage forms the backbone of each strand of DNA.
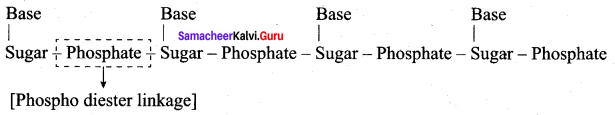
Question 2.
Give the differences between primary and secondary structure of proteins.
Answer:
Primary structure of proteins
- Linear sequence of amino acids
- Composed of peptide bonds formed between amino acids.
- Formed during translation.
- Involved in post – translational modifications.
Secondary structure of proteins
- Folding of the peptide chain into an a-helix and 13-sheet.
- Encompasses hydrogen bonds
- Forms collagen, elastin action, myosin, and keratin – like fibres.
- Involved in forming structures such as cartilages, ligaments, skins etc.
Question 3.
Name the Vitamins whose deficiency cause
- rickets
- scurvy
Answer:
- Vitamin – D deficiency causes rickets disease.
- Vitamin – C deficiency causes scurvy disease.
Question 4.
Write the Zwitter ion structure of alanine.
Zwitter ion structure of alanine
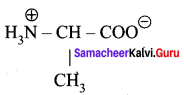
Question 5.
Give any three difference between DNA and RNA.
Answer:
DNA
- It is mainly present in nucleus, mitochondria and chloroplast .
- It contains deoxyribose sugar
- Base pair A = T. G = C
- Double stranded molecules
- Its life time is high
- it is stable and not hydrolysed easily by alkalis
- It can replicate itself
RNA
- It is mainly present in cytoplasm, nucleolus and ribosomes
- It contains ribose sugar
- Base pair A = U. C = G
- Single stranded molecules
- It is Short lived
- It is unstable and hydrolyzed easily by alkalis
- It cannot replicate itself. It is formed from DNA.
Question 6.
Write a short note on peptide bond.
Answer:
1. The amino acids are linked covalently by peptide bonds.
2. The carbonyl group of the first amino acid react with the amino group of the second amino acid to give an amide linkage (- CONH) between these aminoacids. This amide linkage is called peptide bond.
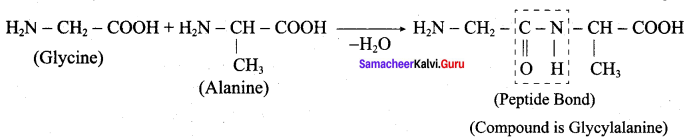
3. The resulting compound is called a dipeptide. Because, two amino acids are inovived for getting one peptide bond.
4. If large number of amino acids combined through peptide bond, the resulting giant molecule is called a protein.
5. The amino end of the peptide is known as N-terminal, while the carboxy end is called C – terminal.
Question 7.
Give two difference between Hormones and vitamins.
Answer:
hormones
- Hormones are produced in the endocrine or ductless glands.
- Hormones are not stored in the body. These are produced as and when required.
- They are effective in low concentration. Their excess or deficiency may cause hormonal disorders.
- Hormones influence the genes to produce specific enzymes required during metabolism.
- Example: Insulin
Vitamins
- Vitamins (except Vitarnin – D) are not produced in the body. Vitamin must be supplied in the diet.
- Vitamins are stored in the body upto certain extent.
- They are needed in small quantity. Excess vitamins are excreted. Their deficiency causes
- malfunctioning called deficiency diseases or avitaminosis.
- They act as co-enzymes and help enzymes to perform their function.
- Example: Vitamin A, B, C, D, E and K
Question 8.
Write a note on den atu ration of proteins.
Answer:
Denaturation of proteins.
1. In general, protein has a unique three – dimensional structure formed by interactions such as disuiphide bond, hydrogen bond, hydrophobic and electrostatic interactions.
2. These interactions can be disturbed when the protein is exposed to a higher temperature in certain chemicals such urea, alternation of pH, ionic strength etc. It leads to the loss of the three – dimensional structure.
3. The process of a protein losing its higher order structure without losing the primary structure, It is called denaturation of protein. When a protein denatures, its biological function is also lost.
4. Since the primary structure is intact, this process can be reversed in certain proteins. This can happen spontaneously upon restoring the original conditions or with the help of special enzymes called cheperons.
5. Example: Coagulation of egg white by action of heat.
Question 9.
What are reducing and non – reducing sugars?
Answer:
1. Reducing sugars:
Those carbonhydrates which contain free aldehyde or ketonic group and reduces Fehling’s solution and Tollen’s reagent are called reducing sugars. All monosacehaides whether aldose or ketone are reducing sugars.
2. Non – reducing sugars:
Cabohydrates which do not reduce Tollen’s reagent and Fehling’s solution are called non – reducing sugars. Example Sucrose. They do not have free aldehyde group.
Question 10.
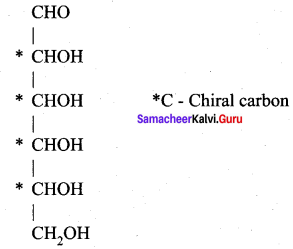
Why carbohydrates are generally optically active?
Answer:
Carbohydrates are generally optically active because they have one or more chiral carbon atoms in their molecules. For example, Glucose has four chiral carbons and therefore it is optically active.
Question 11.
Classify the following into monosaccharides, oligosaccharides and polysaccharldes.
- Starch
- fructose
- sucrose
- lactose
- maltose
Answer:
- Starch – Polysaccharides
- Furctose – Mono saccharides
- Sucrose – Oligo saccharide
- Lactose – Oligo saccharide
- Maltose – Oligo saccharide
Surcose, Lactose and Maltose are typical disaccharides
Question 12.
How are vitamins classified?
Answer:
Vitamins are classified into two groups based on their solubility in water and in fat. They are,
- Water – soluble vitamins
- Oil or fat – soluble vitamins
Water – soluble vitamins – Vitamins which dissolve in water are called water soluble vitamins. Examples – Vitamins of B group and Vitamin C Oil or fat – soluble vitamins: Vitamins which dissolve in oils or fat are called oil or fat – soluble vitamins. Examples – Vitamin A, D, E and K
Question 13.
What are hormones? Give examples.
Answer:
Hormone is an organic substance that is sëcreted by one tissue into the blood stream and induces a physiological response in other tissues. It is an inter cellular signaling molecule. Virtually every process is a complex organism is regulated by one or more hormones. Example, insulin, epinephrine, estrogen, androgen etc.
Question 14.
Write the structure of all possible dipeptides which can be obtained from glycine and atanine.
Answer:
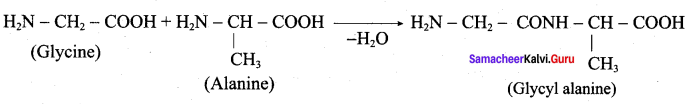
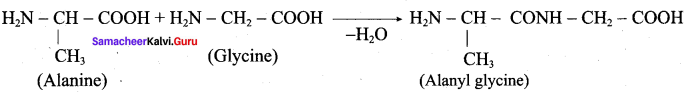
Therefore two dipeptides structures are possible from glycine and alanine. They are
- glycyl alanine and
- Alanyl glycine
Question 15.
Define enzymes.
Answer:
There are many biochemical reactions that occur in our living cells. Digestion of food and harvesting the energy from them, and synthesis of necessary molecules required for various – cellular functions are examples for such reactions. All these reactions are catalysed by special proteins called enzymes.
(or)
Enzymes are bio catalysts produced by the living cells which catalyse many biochemical reactions in animal and plant bodies. They are more specific in their action.
Question 16.
Write the structure of – D (+) glucopyranose.
Answer:
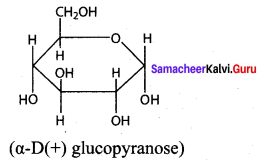
Question 17.
What are different types of RNA which are found in cell?
Answer:
RNA molecules are classified according to their structure and function into three major types.
- Ribosomal RNA (r – RNA)
- Messenger RNA (m – RNA)
- Transfer RNA (t – RNA)
r-RNA:
rRNA is mainly found in cytoplasm and in ribosomes, which contain 60% RNA and 40% protein. Ribosomes are the sites at which protein synthesis takes place.
t – RNA:
tRNA molecules have lowest molecular weight of all nucleic acids. They consist of 73 – 94 nucleotides in a single chain. The function of tRNA is to carry amino acids to the sites of protein synthesis on ribosomes.
m – RNA:
mRNA is present in small quantity and very short lived. They are single stranded, and their synthesis takes place on DNA. The synthesis of mRNA from DNA strand is called transcription. mRNA carries genetic information from DNA to the ribosomes for protein synthesis.
Question 18.
Write a note on formation of – helix.
AnsweR:
1. In the α – helix sub – structure, the aminoacids are arranged in a right handed helical (spiral) structure and are stabilised by the hydrogen bond between the carbonyl oxygen one aminoacid (nth residue) with amino hydrogen of the fifth residue (n + 4th residue)
2. The side chains of the residues protrude outside of the helix. Each turn of an α – helix contains about 3.6 residues and is about 5.4 A long.
3. The amino acid proline produces a line in the helical structure and often called as a helical breaker due to its rigid cyclic structure.
4. Many fibrous proteins such as ct-Keratin in hair, nails, wool, skin and myosin in muscles have α – helix structure. Stretching property of human hair is due to the helical structure of α – keratin in hair.
5. Structure of a-helix.
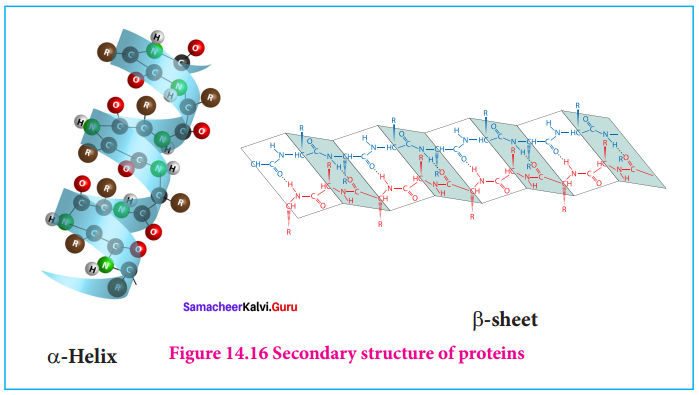
Question 19.
What are the functions of lipids in living organism?
Answer:
- Lipids are the integral component of cell membrane. They are necessary of structural integrity of the cell.
- The main function of triglycerides in animals is as an energy reserve. They yield more energy than carbohydrates and proteins.
- They act as protective coating in aquatic organisms.
- Lipids of connective tissue give protection to internal organs.
- Lipids help in the absorption and transport of fat soluble vitamins.
- They are essential for activation of enzymes such as lipases.
- Lipids act as emulsifier in fat metabolism.
Question 20.
Is the following sugar, D – sugar or L – sugar?
Answer:
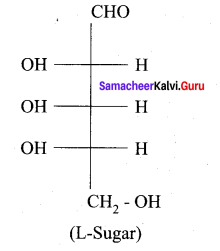
Samacheer Kalvi 12th Chemistry Biomolecules Additional Questions
Samacheer Kalvi 12th Chemistry Biomolecules 1 mark Questions and Answers
I. Choose the best answer.
Question 1.
Which of the following is the most abundant organic compounds in every living organism?
(a) Fats
(b) Proteins
(c) Carbohydrates
(d) Hormones
Answer:
(c) Carbohydrates
Question 2.
What is the general chemical name of carbohydrates?
(a) Poly hydroxy aldehyde or ketones
(b) Poly hydroxy esters
(c) Poly amino acids
(d) Poly carboxylic esters.
Answer:
(a) Poly hydroxy aldehyde or ketones
Question 3.
Which process is utilized in the synthesis of carbohydrates in green plants?
(a) Oxidation
(b) Redox reaction
(c) Photosynthesis
(d) Reduction
Answer:
(c) Photosynthesis
Question 4.
Which of the following compounds are optically active?
(a) Glycine
(b) Carbohydrates
(c) Ethanol
(d) Meso tartaric acid
Answer:
(b) Carbohydrates
Question 5.
Which of the following is optically inactive?
(a) 2 – butanol
(b) Glyceraldehyde
(c) Glucose
(d) Meso tartaric acid
Answer:
(d) Meso tartaric acid
Question 6.
How many isomers are possible for glucose that have 4 asymmetic carbon atoms?
(a) 8 isomers
(b) 16 isomers
(c) 2 isomers
(d) 4 isomers
Answer:
(b) 16 isomers
Question 7.
How many asymmetric carbon atoms are in glucose?
(a) 4
(b) 3
(c) 2
(d) 1
Answer:
(a) 4
Question 8.
Which of the following rotates the plane polarised light in clockwise direction?
(a) L(-) Glucose
(b) D (glucose)
(c) L – fructose
(d) L – Glyceraldehyde
Answer:
(b) D (glucose)
Question 9.
Which one of the following is levorotatory?
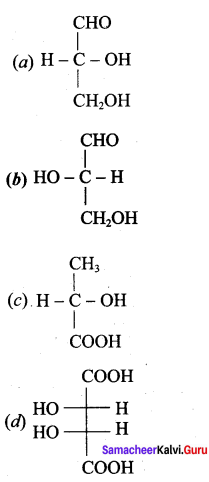
Answer:
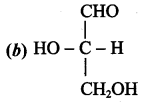
Question 10.
Which one of the following is not a monosaccharide?
(a) Fructose
(b) Ribose
(c) Erythrose
(d) Maltose
Answer:
(d) Maltose
Question 11.
Which one of the following is a monossachande?
(a) Glucose
(b) Maltose
(c) Surcose
(d) Cellulose
Answer:
(a) Glucose
Question 12.
The number of carbon atoms present in Erythrose is
(a) 6
(b) 4
(c) 3
(d) 5
Answer:
(b) 4
Question 13.
What is the amount of glucose present in human blood?
(a) 150 mg/dI
(b) 50 mg/dl
(c) 100 mg/dl
(d) 1000 mg/dl
Answer:
(c) 100 mg/dl
Question 14.
Which one of the following is called blood sugar?
(a) Erythrose
(b) Ribose
(c) Ribulose
(d) Glucose
Answer:
Question 15.
Acid hydrolysis of starch at high temperature and pressure produces
(a) fructose
(b) glucose
(c) both fructose and glucose
(d) maltose
Answer:
(b) glucose
Question 16.
The other name of glucose is …………
(a) dextrose
(b) blood sugar
(c) aldohexose
(d) all the above
Answer:
(d) all the above
Question 17.
Which one is formed as major product when glucose is on reduction with concentrated HI and red P at 3 73K?
(a) 2 – iodohexane
(b) 3 – iodohexane
(c) n – hexane
(d) 4 – iodohexane
Answer:
(c) n – hexane
Question 18.
Which one of the product is formed when glucose reacts with bromine water?
(a) n – hexane
(b) Gluconic acid
(c) Saccharic acid
(d) Hexanoic acid
Answer:
(c) Saccharic acid
Question 19.
Which one of the following is formed when glucose react with Conc. HNO3?
(a) Gluconic acid
(b) Glutaric acid
(c) Saccharic acid
(d) Hexanoic acid
Answer:
(c) Saccharic acid
Question 20.
Which one of the following will reduce Tollen’s reagent and Fehling’s solution?
(a) Glucose
(b) Fructose
(c) Sucrose
(d) Maltose
Answer:
(a) Glucose
Question 21.
Which of the following form pentacetate with acetic anhydide?
(a) Glucose
(b) Fructose
(c) Lactose
(d) Both a & b
Answer:
(d) Both a & b
Question 22.
Which one of the reagent does not react with glucose?
(a) Acetic anhydride
(b) Tollen’s reagent
(c) Sodium bi suiphite
(d) Bromine water
Answer:
(c) Sodium bi suiphite
Question 23.
The specific rotation of pure α and β (D) glucose are respectively.
(a) 18.7°, 112°
(b) 112°, 18.7°
(c) 90°, 90°
(d) 120°, 20°
Answer:
(b) 112°, 18.7°
Question 24.
Sugar differing in configuration at an asymmetric centre is known as ……………….
(a) epimers
(b) isomers
(c) anomers
(d) monomers
Answer:
(a) epimers
Question 25.
Which enzyme is utilised in the conversion of galactose to glucose?
(a) Maltose
(b) Epimerase
(c) Invertase
(d) Zymase
Answer:
(b) Epimerase
Question 26.
The other name of fructose is ……………
(a) Ketohexose
(b) fruit sugar
(c) levulose
(d) all the above
Answer:
(d) all the above
Question 27.
Hydrolysis of mutin in acidic medium gives ……………
(a) glucose
(b) fructose
(c) both a & b
(d) maltose
Answer:
(b) fructose
Question 28.
Invert sugar is a mixture of equal amount of ……………..
(a) lactose + maltose
(b) diastose + galactose
(c) glucose + fructose
(d) starch + cellulose
Answer:
(c) glucose + fructose
Question 29.
Which enzyme is used in the conversion of sucrose into glucose and fructose?
(a) Zymase
(b) Invertase
(c) Diastase
(d) Maltase
Answer:
(b) Invertase
Question 30.
Which one of the following is the sweetest of all known sugars?
(a) Lactose
(b) Glucose
(c) Fructose
(d) Sucrose
Answer:
(c) Fructose
Question 31.
Which is the product formed when fructose undergoes partial reduction with sodium amalgam and water?
(a) Sorbital + mannitol
(b) D – mannose + D – galactose
(c) Gluconic acid + saccharic acid
(d) Aldehyde + ketone
Answer:
(a) Sorbital + mannitol
Question 32.
Which one of the following reagent is used to convert fructose into sorbitol and mannitol?
(a) LiAlH4
(b) Hl / Red P
(c) Na / Hg
(d) Conc. HNO3
Answer:
(c) Na / Hg
Question 33.
Fructose on oxidation with concentrated nitric acid gives
(a) glyceric acid + oxalic acid
(b) glycoffic acid + tartaric acid
(c) tartronic acid + mesoxalic acid
(d) acetic acid + hexanoic acid
Answer:
(b) glycoffic acid + tartaric acid
Question 34.
How many asymmetric carbon atoms are present in fructose?
(a) 4
(b) 3
(c) 2
(d) 6
Answer:
(b) 3
Question 35.
Two monosaccharides are linked by to form a disaccharide.
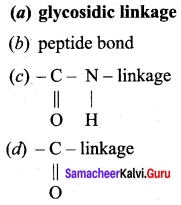
Answer:
(a) glycosidic linkage
Question 36.
Which of the following is not an example of disaccharide?
(a) Sucrose
(b) Lactose
(c) Maltose
(d) Cellulose
Answer:
(d) Cellulose
Question 37.
The enzyme that catalyses the hydrolysis of sucrose to glucose and fructose is …………….
(a) zymase
(b) invertase
(c) diastase
(d) maltase
Answer:
(b) invertase
Question 38.
Which one of the following contains a mixture of glucose, fructose and sucrose?
(a) Sugarcane
(b) Bectroot
(c) Honey
(d) Mango
Answer:
(c) Honey
Question 39.
Consider the following statements
(i) in sucrose, C1 of a – D – glucose is joined to C2 of D – fructose.
(ii) Two monosaccharides are linked by glycosidic linkage.
(iii) In sucrose, C2 of a – D – glucose is joined to C1 of D – fructose.
Which of the above statement is / are correct?
(a) (iii) only
(b) (i) & (ii)
(c) (ii) only
(d) (i) & (iii)
Answer:
(b) (i) & (ii)
Question 40.
Which one of the following is an example of non – reducing sugar?
(a) Glucose
(b) Dextrose
(c) Lactose
(d) Sucrose
Answer:
(d) Sucrose
Question 41.
Which one of the following on hydrolysis give galactose and glucose?
(a) Maltose
(b) Cellulose
(c) Lactose
(d) Sucrose
Answer:
(c) Lactose
Question 42.
Which one of the following is called milk sugar?
(a) Lactose
(b) Glucose
(c) Sucrose
(d) Raffinose
Answer:
(a) Lactose
Question 43.
Which of the following is the major source of maltose?
(a) Honey
(b) Apple
(c) Sprouting barley
(d) Grapes
Answer:
(c) Sprouting barley
Question 44.
Which one is produced during digestion of starch by the enzyme α – amylase?
(a) Maltose
(b) Glucose
(c) Fructose
(d) Lactose
Answer:
(a) Maltose
Question 45.
Consider the following statements
(I) Maltose consists two molecules of α – D glucose units linked by an α – 1, 4 glycosidic bond.
(ii) Maltose act as non-reducing sugar.
(iii) Maltose is produced during digestion of cellulose by the enzyme a-amylase.
Which of the above statement is / are not correct?
(a) (i) only
(b) (i) & (iii)
(c) (iii) only
(d) (ii) & (iii)
Answer:
(d) (ii) & (iii)
Question 46.
Which one of the following is hetero polysaccharide?
(a) Starch
(b) Heparin
(c) Cellulose
(d) Glycogen
Answer:
(b) Heparin
Question 47.
Which one of the following is a homopolysaccharide?
(a) Hyaluronic acid
(b) Heparin
(c) Both (a) & (b)
(d) Starch
Answer:
(d) Starch
Question 48.
Consider the following statements
(I) Starch contains 80% of amylase and about 20% amylopectin.
(ii) Polysaccharides are called sugars.
(iii) Lactose act as a reducing agent.
Which of the above statement is / are not correct?
(a) (i) & (ii)
(b) (iii)
(c) (ii) & (iii)
(d) (ii) only
Answer:
(a) (i) & (ii)
Question 49.
Which one of the following gives blue colour with amylose and purple colour with amylopectin?
(a) Tollen’s reagent
(b) Fehling’s solution
(c) Iodine solution
(d) Bromic water
Answer:
(c) Iodine solution
Question 50.
Which colour is formed when amylose is treated with iodine solution?
(a) Purple
(b) Red
(c) Blue
(d) Violet
Answer:
(c) Blue
Question 51.
Which colour is formed when amylopectin is treated with iodine solution?
(a) Purple
(b) Blue
(c) Green
(d) Red
Answer:
(a) Purple
Question 52.
Which one of the following is the major constituent of plant cell walls?
(a) Starch
(b) Cellulose
(c) Glycogen
(d) Amylose
Answer:
(b) Cellulose
Question 53.
Consider the following statements
(i) Cellulose is a straight chain polysaccharide.
(ii) The glucose molecules in cellulose arc linked by β(1, 4) glycosidic bond.
(iii) Cotton is almost pure starch.
Which of the above statement is / are correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i) & (ii)
Answer:
(d) (i) & (ii)
Question 54.
Which of the following enzyme can hydrolyse the cellulose?
(a) Invertase
(b) Glycosidase
(c) Zymase
(d) Diastase
Answer:
(b) Glycosidase
Question 55.
Which one of the following is called gun cotton?
(a) Nitrated ester of cellulose
(b) Cellulose acetate
(c) Glyceryl trinitrate
(d) Trinitrotoluene
Answer:
(a) Nitrated ester of cellulose
Question 56.
Which one of the following is called animal starch?
(a) Cellulose
(b) Glycogen
(c) Lactose
(d) Fat
Answer:
(b) Glycogen
Question 57.
Consider the following statements
(i) The excessive glucose in the body is stored in the form of arnylose and amylopectin.
(ii) Glycogeri is present in the liver and muscle of animals.
(iii) Protein is stored in the body as glycogen and in plant as starch.
Which of the above statement is / are not correct?
(a) (¡) & (iii)
(b) (ii) & (iii)
(c) (i) only
(d) (iii) only
Answer:
(d) (iii) only
Question 58.
Which one of the following is stored in the body as glycogen and in plant as starch?
(a) Protein
(b) Vitamin
(c) Fat
(d) Carbohydrates
Answer:
(d) Carbohydrates
Question 59.
Which one of the following act as shock absorber and lubricant?
(a) Glycosamino glycans
(b) Glycogen
(c) Cellulose nitrate
(d) Rayon explosive
Answer:
Question 60.
Which biomolecule is the most abundant in all living organisms?
(a) Carbohydrates
(b) Vitamins
(c) Hormones
(d) Proteins
Answer:
(d) Proteins
Question 61.
Which of the following is mainly present in proteins?
(a) 3 – keto acid
(b) 1 – amino acid
(c) α, β – ketol
(d) amide and acids
Answer:
(b) 1 – amino acid
Question 62.
Which of the amino acid is optically inactive?
(a) Alanine
(b) Valine
(c) Glycine
(d) Proline
Answer:
(c) Glycine
Question 63.
Proteins are generally …………..
(a) poly amides
(b) polyesters
(c) polymer
(d) poly peptide
Answer:
(d) poly peptide
Question 64.
Which one of the following is an example for fibrous protein?
(a) Myoglobin
(b) Insulin
(c) Keratin
(d) Enzymes
Answer:
(c) Keratin
Question 65.
Which one of the following is an example for globular protein?
(a) Kerating
(b) Myoglobin
(c) Collagen
(d) Etastin
Answer:
(b) Myoglobin
Questin 66.
Consider the following statement
(i) The amino acids arc linked electro valently by peptide bonds in proteins.
(ii) Fibrous proteins are linear molecules similar to fibres.
(iii) Globular proteins have a linear shape.
Which of the above statement is / are not correct?
(a) (iii) only
(b) (i) & (iii)
(c) (ii) & (iii)
(d) (ii) only
Answer:
(b) (i) & (iii)
Question 67.
Consider the following statement
Answer:
(I) The relative arrangement of amino acids in the polypeptide chain called the secondary structure of protein.
(ii) α – Helix and β – strands are two most common sub structures formed by proteins.
(iii) α – Helix and β – strands further folds to form the three dimensional arrangement in tertiary structure of proteins.
Which of the above statement is / are correct?
(a) (i) only
(b) (ii) & (iii)
(c) (i) & (iii)
(d) (ii) only
Answer:
(b) (ii) & (iii)
Question 68.
Which of the following act as structural backbones?
(a) Keratine, collagen
(b) Myoglobin, insulin
(c) Glycine, proline
(d) Alanine, cysteine
Answer:
(a) Keratine, collagen
Question 69.
Which protein control the glucose level in the blood?
(a) Kerating, collagen
(b) Insulin, glucagon
(c) Glycine, proline
(d) Alanine, myoglobin
Answer:
(b) Insulin, glucagon
Question 70.
Which one of the following act as catalyst in the interconversion of carbonic acid to water and carbondioxide?
(a) Lactose
(b) Carbonic anhydrase
(c) Glycosidase
(d) Invertase
Answer:
(b) Carbonic anhydrase
Question 71.
Which enzyme catalyses the hydrolysis of sucrose to fructose and glucose?
(a) Lactase
(b) invertase
(c) Sucrase
(d) Zymase
Answer:
(c) Sucrase
Question 72.
Lactase enzyme hydrolyses the lactose into its constituent as …………..
(a) glucose, fructose
(b) glucose, galactose
(c) fructose only
(d) glucose only
Answer:
(b) glucose, galactose
Question 73.
Consider the following statement
(i) Lipids are the principal components of cell membranes including cell walls.
(ii) Enzymes are biocatalysts that catalyse a specific biochemical reaction.
(iii) Carbonic anhydrase enzyme catalyses the hydrolysis of sucrose to fructose and glucose.
Which of the above statement is / are correct?
(a) (i) only
(b) (iii) only
(c) (i) & (iii)
(d) (ii) & (iii)
Answer:
(c) (i) & (iii)
Question 74.
Which one help in the absorption and transport of fat soluble vitamins?
(a) Lipids
(b) Protein
(c) Enzyme
(d) Water
Answer:
(a) Lipids
Question 75.
Which one act as emulsifier in fat metabolism?
(a) Enzymes
(b) Fats
(c) Lipids
(d) Proteins
Answer:
(c) Lipids
Question 76.
Which one of the following is fat soluble vitamin?
(a) Vitamin B1
(b) Vitamin B6
(c) Vitamin C
(d) Vitamin A
Answer:
(d) Vitamin A
Question 77.
Which one of the following is a water soluble vitamin?
(a) Vitamin A
(b) Vitamin D
(c) Vitamin C
(d) Vitamin K
Answer:
(c) Vitamin C
Question 78.
Which one of the following deficient disease of Vitamin A?
(a) Cheilosis
(b) Xerophthalmia
(c) Convulsions
(d) Perncious Anaemia
Answer:
(b) Xerophthalmia
Question 79.
Which Vitamin deficiency leads to cheilosis?
(a) Vitamin B12
(b) Vitamin B6
(c) Vitamin B2
(d) Vitamin B5
Answer:
(c) Vitamin B2
Question 80.
Which one of the following vitamin deficiency leads to Rickets?
(a) Vitamin A
(b) Vitamin B1
(c) Vitamin C
(d) Vitamin D
Answer:
(d) Vitamin D
Question 81.
Which vitamin deficiency leads to Hair loss, muscle pain?
(a) Biotin
(b) Niacin
(c) Riboflavin
(d) Thiamine
Answer:
(a) Biotin
Question 82.
The deficiency of Vitamin B12 leads to the disease ………………..
(a) convulsions
(b) beriberi
(c) Pernicious anaemia
(d) pellagra
Answer:
(c) Pernicious anaemia
Question 83.
Which of the following is the chemical name of vitamin B12?
(a) Folic acid
(b) Cobalamin
(c) Pyridoxime
(d) Riboflavin
Answer:
(b) Cobalamin
Question 84.
Night blindness and kertinisation of skin is the chemical name of Vitamin B12?
(a) vitamin B1
(b) vitamin C
(c) vitamin A
(d) vitamin B12
Answer:
(c) vitamin A
Question 85.
Which vitamin deficiency leads to the disease megaloblastic anaemia?
(a) Vitamin B9
(b) Vitamin B6
(c) Vitamin B12
(d) Vitamin B2
Answer:
(a) Vitamin B9
Question 86.
Which one of the following is rich in liver oil, carrot, mango and papaya?
(a) Vitamin B1
(b) Vitamin C
(c) Vitamin A
(d) vitamin D
Answer:
(c) Vitamin A
Question 87.
Which of the vitamin deficiency leads to photosensitive dermatitis (or) pellagra?
(a) Vitamin B5
(b) Vitamin B6
(c) Vitamin B3
(d) Vitamin D
Answer:
(c) Vitamin B3
Question 88.
Depression, Hair loss, muscle pain are due to the deficiency of vitamin ……………..
(a) A
(b) B12
(c) B2
(d) B7
Answer:
(d) B7
Question 89.
The chemical name of vitamin B9 is …………….
(a) biotin
(b) folic acid
(c) niacin
(d) thiamin
Answer:
(b) folic acid
Question 90.
Which of the following is rich in citrus, fruits, tomato, amia and leafy vegetables?
(a) Vitamin C
(b) Vitamin E
(c) Vitamin A
(d) Vitamin D
Answer:
(a) Vitamin C
Question 91.
Consider the following statement
(i) Vitamin D functions in the adsorption and maintenance of calcium.
(ii) Vitamin E act as an antioxidant.
(iii) Vitamin C functions in blood clotting.
Which of the above statement is / are correct?
(a) (iii) only
(b) (ii) & (iii)
(c) (i) & (ii)
(d) (i) only
Answer:
(c) (i) & (ii)
Question 92.
Which vitamin is rich in cotton seed oil, sunflower oil, wheat germ oil and all vegetable oils?
(a) Vitamin C
(b) Vitamin E
(c) Vitamin A
(d) Vitamin D
Answer:
(b) Vitamin E
Question 93.
Which vitamin deficiency leads to the disease osteomalacia?
(a) Vitamin D
(b) Vitamin A
(c) Vitamin C
(d) Vitamin K
Answer:
(a) Vitamin D
Question 94.
Which one of the following is mainly required for blood clotting?
(a) Vitamin E
(b) Vitamin B12
(c) Vitamin C
(d) Vitamin K
Answer:
(d) Vitamin K
Question 95.
Consider the following statement
(i) Nucleic acid are biopolymers of nucleotides.
(ii) Controlled hydrolysis of DNA and RNA yield 3 components namely a nitrogeneous base, a pentose sugar and sulphate group.
(iii) DNA and RNA are the molecular repositories that carry genetic information in every organism.
Which of the above statement is / are correct?
(a) (i) only
(b) (ii) only
(c) (i) & (iii)
(d) (ii) & (iii)
Answer:
(c) (i) & (iii)
Question 96.
Which one of the following is found in cytoplasm and in nbosomers which contain 60% RNA and 40% protein.
(a) Ribosomal RNA
(b) Messenger RNA
(c) Transfer RNA
(d) DNA
Answer:
(a) Ribosomal RNA
Question 97.
Consider the following statement .
(i) Ribosomes are the sites at which protein synthesis takes place.
(ii) Messenger RNA carried genetic information from DNA to the ribosomes for protein synthesis.
(iii) t RNA consist of 20 – 40 nucleotides in a single chain.
Which of the above statement is / are not correct?
(a) (i) only
(b) (i) & (ii)
(c) (iii) only
(d) (ii) & (iii)
Answer:
(c) (iii) only
Question 98.
What is the name of the process of synthesis of mRNA from DNA strand?
(a) Transpiration
(b) Transcription
(c) Transformation
(d) Trans esterification
Answer:
(b) Transcription
Question 99.
Consider the following statement
(i) DNA mainly present in cytoplasm, nucleolus and ribosomes.
(ii) RNA is stable and not hydrolysed easily by alkalis.
(iii) DNA can replicate itself.
Which of the above statement is / are correct?
(a) (iii) only
(b) (i) only
(c) (i) & (ii)
(d) (ii) & (iii)
Answer:
(c) (i) & (ii)
Questin 100.
Who invented DNA finger printing?
(a) Sir Alec Jeffrey
(b) Rosalind Franklin
(c) Watson and Crick
(d) Maurice Wilkins
Answer:
(a) Sir Alec Jeffrey
Question 101.
Which one of the following can act as energy carriers?
(a) GTN
(b) ATP
(c) FAD
(d) Cyclic AMP
Answer:
(b) ATP
Questin 102.
Adenosine 3’, 5’- cyclic monophosphate a chemical messenger is otherwise called …………….
(a) ATP
(b) cyclic ADP
(c) cyclic AMP
(d) 3’P – ADP
Answer:
(c) cyclic AMP
Question 103.
Consider the following statement
(i) Endocrine glands make hormones.
(ii) Hormones may be classified as either protein (or) steroids
(iii) Hormones are intracellular signalling molecule.
Which of the above statement is / are not correct?
(a) (i) only
(b) (ii) & (iii)
(c) (iii) only
(d) (i) & (iii)
Answer:
(c) (iii) only
Question 104.
Which one of the following is a steroid?
(a) Insulin
(b) Epinephrine
(c) mutin
(d) Estrogen
Answer:
(d) Estrogen
Question 105.
Which one of the following is a protein hormone?
(a) Insulin
(b) Androgen
(c) Cortisol
(d) Estrogen
Answer:
(a) Insulin
Question 106.
The nucleic acid base having two possible binding sites is …………….
(a) thymine
(b) cytosine
(c) guanine
(d) adenine
Answer:
(c) guanine
Question 107.
DNA multiplication is called ………….
(a) transcription
(b) transformation
(c) transduction
(d) replication
Answer:
(d) replication
Question 108.
Insulin is a protein which plays the role of ……………
(a) an antibody
(b) a hormone
(c) an enzyme
(d) a transporting agent
Answer:
(b) a hormone
Question 109.
Which metal is present in Vitamin B12?
(a) Ca (II)
(b) Zn (II)
(c) Fe (II)
(d) Co (III)
Answer:
(d) Co (III)
Question 110.
The helical structure of protein is stablized by ………………
(a) oxygen bonds
(b) peptide bonds
(c) dipeptide bonds
(d) hydrogen bonds
Answer:
(d) hydrogen bonds
Question 111.
The cell membranes are mainly composed of ………….
(a) carbohydrates
(b) proteins
(c) phospholipids
(d) fats
Answer:
(c) phospholipids
Question 112.
Which one of the following is a polysaccharide?
(a) Nylon
(b) Amylose
(c) Ribose
(d) Polyethene
Answer:
(b) Amylose
Question 113.
Ribose ¡san example of …………….
(a) keto hexose
(b) aldohexose
(c) aldo pentose
(d) disaccharide
Answer:
(c) aldo pentose
Question 114.
Sucrose molecule is made up of …………..
(a) a gluco pyranose and fructo pyranose
(b) a glyco pyranose and fructo furanose
(c) a gluco furanose and fructo pyranose
(d) a gluco furanose and fructo furanose
Answer:
(b) a glyco pyranose and fructo furanose
Question 115.
A nucleotide consists of ……………….
(a) base and sugar
(b) base and phosphate
(c) sugar and phosphate
(d) base, sugar and phosphate
Answer:
(d) base, sugar and phosphate
Question 116.
Which of the following is responsible for heredity character?
(a) DNA
(b) RNA
(c) Proteins
(d) Hormones
Answer:
(a) DNA
Question 117.
The base adenine present in …………….
(a) DNA only
(b) RNA only
(c) Both DNA & RNA
(d) Protein
Answer:
(c) Both DNA & RNA
Question 118.
The protein which maintains the blood sugar level in the human body is …………..
(a) haemoglobin
(b) oxytocin
(c) insulin
(d) ptyalin
Answer:
(c) insulin
Question 119.
Ascorbic acid is a …………
(a) vitamin
(b) enzyme
(c) protein
(d) hormone
Answer:
(a) vitamin
Question 120.
Which of the following is not a constitutent of RNA?
(a) Ribose
(b) Phosphate
(c) Adenine
(d) Pyridine
Answer:
(d) Pyridine
Question 121.
Which one is fouñd in ATP ribonucleotide?
(a) Guanine
(b) Uracil
(c) Adenine
(d) Inulin
Answer:
(c) Adenine
Question 122.
Which substance is not present in nucleic acid?
(a) Cytosine
(b) Adenine
(c) Thymine
(d) Guanidine
Answer:
(d) Guanidine
Question 123.
In nucleic acid, the correct sequence is ……………..
(a) base – phosphate sugar
(b) phosphate – base – sugar
(c) sugar – base – phosphate
(d) base – sugar – phosphate
Answer:
(d) base – sugar – phosphate
Question 124.
The double helical structure of DNA was proposed by ……………….
(a) Watson and Crick
(b) Meicher
(c) Emil Fischer
(d) Khorana
Answer:
(a) Watson and Crick
Question 125.
Which substance is not present in nucleic acid?
(a) Cytosine
(b) Adenine
(c) Thymine
(d) Guanidine
Answer:
(d) Guanidine
Question 126.
In DNA, the complementary bases are ……………….
(a) Uracil and adenine; cytosine and guanine
(b) Adenine and thymine; guanine and cytosine
(c) Adenine and guanine; thymine and cytosine
(d) adenine and guanine; thymine and uracil
Answer:
(b) Adenine and thymine; guanine and cytosine
Question 127.
The structure of DNA is …………….
(a) linear
(b) single helix
(c) double helix
(d) triple helix
Answer:
(c) double helix
Question 128.
A gene is a segment of molecule of ……………
(a) DNA
(b) m – RNA
(c) t – RNA
(d) protein
Answer:
(a) DNA
Question 129.
The deficiency of vitamin C causes …………….
(a) scurvy
(b) rickets
(c) pyrrohea
(d) pellagra
Answer:
(a) scurvy
Question 130.
Which sugar is present in DNA?
(a) Deoxyribose
(b) Ribose
(c) D – fructose
(d) D – glucose
Answer:
(a) Deoxyribose
Question 131.
The base present ¡n DNA but not in RNA is …………..
(a) guanne
(b) adenine
(c) uracil
(d) thymine
Answer:
(d) thymine
Question 132.
Mutation of DNA occurs due to changes in the sequence of one of the following.
(a) Bases
(b) Ribose units
(c) Phosphate units
(d) Sugar units
Answer:
(a) Bases
Question 133.
Blood calcium level can be increased by the administration of ……………….
(a) glucogon
(b) calcitonin
(c) thyroxine
(d) paratharmone
Answer:
(d) paratharmone
Question 134.
The first hormone chemically synthesised in the laboratory is …………….
(a) cortisone
(b) insulin
(c) adrenaline
(d) eastrone.
Answer:
(b) insulin
Question 135.
RNA is different from DNA because RNA contains ……………
(a) Ribose sugar and tymine
(b) Ribose sugar and uracil
(c) Doxyribose sugar and thymine
(d) Deoxy ribose sugar and uracil
Answer:
(b) Ribose sugar and uracil
Question 136.
The hormone that helps in the conversion of glucose to glycogen is ……………..
(a) adrenaline
(b) insulin
(c) cortisone
(d) bile acid
Answer:
(b) insulin
Question 137.
Enery is stored in our body in the form of ………….
(a) ATP
(b) ADP
(c) Fats
(d) carbohydrates
Answer:
(a) ATP
Question 138.
Nucleic acid is a polymer of …………….
(a) Nucleosides
(b) a – aminoacids
(c) nucleotides
(d) glucose
Answer:
(c) nucleotides
Question 139.
Which one of the following is named as peptides?
(a) Esters
(b) Salts
(c) Amides
(d) Ketones
Answer:
(c) Amides
Question 140.
Irreversible precipitation of proteins is called ……………
(a) denaturation
(b) hydrolysis
(c) transformation
(d) trans esterification
Answer:
(a) denaturation
Question 141.
Which of the following is not an essential amino acid?
(a) Valine
(b) Lysinc
(c) Histidine
(d) Glycine
Answer:
(d) Glycine
Question 142.
Proteins are hydrolysed by enzymes into …………….
(a) dicarboxylic acid
(b) hydroxy acids
(c) amino acids
(d) aromatic acids
Answer:
(c) amino acids
Question 143.
Which one of the protein transports oxygen in the blood stream?
(a) Myoglobin
(b) Insulin
(c) Albumin
(d) Haemoglobin
Answer:
(d) Haemoglobin
Question 144.
Enzymes in the living systems …………..
(a) provide energy
(b) provide immunity
(c) catalyse biological process
(d) transport oxygen
Answer:
(c) catalyse biological process
Question 145.
Which compound can exist in a dipolar state?
(a) C6H5 CH2 CH (N = CH2) COOH
(b) (CH3)2 CH – CH (NH2) COOH
(c) C6H5CONH CH2 COOH
(d) HOOC – CH2 – CH2 – CO – COOH
Answer:
(b) (CH3)2 CH – CH (NH2) COOH
Question 146.
Haemoglobin is …………….
(a) an enzyme
(b) a globular protein
(c) a vitamin
(d) carbohydrate
Answer:
(b) a globular protein
Question 147.
The number of essential amino acid in man is …………….
(a) 8
(b) 10
(c) 20
(d) 18
Answer:
(b) 10
Question 148.
Which one of the biomolecule is insoluble in water?
(a) Keratin
(b) Haemoglobin
(c) Ribonuclease
(d) Aclenine
Answer:
(a) Keratin
Question 149.
Which of the following is used in our body as a fuel for muscles and nerves and to build and repair body tissues?
(a Cane sugar
(b) Fructose
(c) Proteins
(d) Glucose
Answer:
(c) Proteins
Question 150.
The bond that determines the secondary structure of proteins is …………..
(a) coordinate bond
(b) covalent bond
(c) hydrogen bond
(d) peptide bond
Answer:
(c) hydrogen bond
Question 151.
Which of the following monosaccharide is a pentose?
(a) Galactose
(b) Glucose
(c) Fructose
(d) Arabinose
Answer:
(d) Arabinose
Question 152.
Which of the following is a carbohydrate?
(a) Leucine
(b) Albumin
(c) Inulin
(d) Maltase
Answer:
(c) Inulin
Question 153.
Glucose gives silver mirror with Tollens reagent. it shows the presence of ……………..
(a) an acidic group
(b) an alcoholic group
(c) a ketonic group
(d) an aldehydic group
Answer:
(d) an aldehydic group
Question 154.
The compound which does not contain an asymmetric carbon atom is …………….
(a) glyceraldehyde
(b) glycine
(c) glucose
(d) fructose
Answer:
(b) glycine
Question 155.
Which one of the following compounds is found abundantly in nature?
(a) Fructose
(b) Starch
(c) Glucose
(d) Cellulose
Answer:
(d) Cellulose
Question 156.
Blood sugar is the same as ……………
(a) glucose
(b) galactose
(c) glycogen
(d) fructose
Answer:
(a) glucose
Question 157.
Which of the following is an aldohexose?
(a) Sucrose
(b) Cellulose
(c) Glucose
(d) Raffinose
Answer:
(c) Glucose
Question 158.
Glucose and mannose are …………..
(a) epimers
(b) anomers
(c) keto hexoses
(d) disaccharides
Answer:
(a) epimers
Question 159.
Which of the following is the sweetest sugar?
(a) Glucose
(b) Fructose
(c) Lactose
(d) Sucrose
Answer:
(b) Fructose
Question 160.
In fructose, the possible optical isomers are …………..
(a) 12
(b) 16
(c) 8
(d) 4
Answer:
(c) 8
Question 161.
Which one of the following is not used to convert glucose into gluconic acid?
(a) Br2 water
(b) Cone. HNO3
(c) Tollen’s reagent
(d) Fehling’s solution.
Answer:
(b) Cone. HNO3
II. Fill in the blanks.
- Chemically, carbohydrates are defined as …………. or …………. with a general formula
- …………. are synthesised by green leaves during photo synthesis.
- Almost all …………. are optically active as they have one or more chiral carbon.
- …………. are carbohydrates that cannot be hydrolysed further and are also called simple sugars.
- Erythrose is an example of ………….
- Glyceraldehyde is an example of …………. in monosaccharides.
- Glucose in human blood about …………. and it also known as ………….
- Ribulose is an example of ………….
- Glucose solution is optically active and it rotates the plane polarised light in …………. direction and so it called
- Glucose when oxidised with con.HNO3 gives ………….
- The reaction of glucose with Tollents reagent or Fehlmg’s solution confirms the presence of …………. group in glucose.
- The exact special arrangement of – OH groups in glucose was given by ………….
- The two isomers differ only in the configuration of C1 carbon are called ………….
- The cyclic structure of glucose with 5 carbon and one oxygen atom is called ………….
- The slow interconversion of α – D glucose and β – D glucose via open chain form under equilibrium is called ………….
- Sugars differing in configuration at an asymmetric centre is known as ………….
- …………. is present abundantly in fruts and hence it is also called fruit sugar.
- The solution having equal amount of glucose and fructose is termed as ………….
- Partial reduction of fructose with sodium amalgam and water produces …………. and …………. which are at second carbon.
- The reaction sodium amalgam and water with fructose confirms the presence of ………….
- The cyclic form of fructose is called ………….
- Disaccharides have general formula ………….
- In disaccharides, two monosaccharides are linked by …………. called ………….
- …………. is primary mixture of glucose, fructose and sucrose.
- Sucrose is also called …………. sugar.
- …………. is produced during digestion of starch by the enzyme a-amylase
- Starch contains about 20% …………. and about 80% of .
- Starch is used for …………. in plants.
- Cotton is almost pure ………….
- …………. is the storage polysaccharides of animals.
- …………. act as shock absorber and lubricant
- Proteins are polymers of ………….
- Orinithine and citrulline are called ………….
- At a specific pH value the net charge of an amino acid in neutral is called ………….
- Except …………. all other amino acids are optically active.
- In proteins, the amino acids are linked covalently by ………….
- The process of a protein, losing its higher order structure without losing the primary structure is called ………….
- Proteins such as …………. , …………. act as structural back bones.
- …………. and …………. controls the glucose level in the blood.
- …………. are biocatalysts that catalyse a specific biochemical reaction.
- …………. act as protective coating in aquatic organisms.
- Lipids act as …………. in fat metabolism.
- …………. help in the absorption and transport of fat soluble vitamins.
- Vitamin A, D, E and K …………. are vitamins.
- The chemical name of Vitamin A is ………….
- …………. deficiency leads to the disease cheilosis.
- …………. deficiency leads to the disease pellagra.
- …………. is a part of coenzyme A in carbohydrate protein and fat metabolism.
- …………. is rich in mushroom, avocada, egg yolk, sunflower oil.
- …………. deficiency leads to pernicious Anaemia.
- All citrus fruits and amla are rich in vitamin ………….
- …………. functions in blood clotting.
- Nucleic acids are bio polymers of ………….
- Both DNA and RNA have two major purine bases …………. and ………….
- The recurring deoxyribonucleotie units of DNA contains …………. and the ribonucleotide units of RNA contain
- The molecule with the phosphate group is called a ………….
- The specific association of the two chains of the double helix in DNA is known as ………….
- t RNA molecule consists of …………. nucleotides in a single chain
- The synthesis of mRNA from DNA strand is called ………….
- …………. carries genetic information from DNA to the ribosomes for protein synthesis.
- …………. was first invented by Sir Alec Jeifry.
Answer:
- polyhydroxy aldehydes, ketoses, C(H2O)
- Carbohydrates
- carbohydrates
- Monosaccharides
- monosaccharide
- aldotriose
- 100 mg/dI, blood sugar
- ketopentose
- clockwise, dextrose
- glucaric acid (or) saccharic acid
- aldehyde
- Emil Fischer
- anomers
- pyranose form
- mutarotation
- epimers
- fructose
- invert sugar
- sorbitol, mannitol, epimers
- keto group
- furanose form
- Cn(H2O)n-1
- oxide, linkage, glycosidic linkage
- honey.
- invert (or) non reducing
- maltose
- amylose, amylopectin
- energy storage
- cellulose
- glycogen
- hyaluronate (or) glycosaminoglycans
- α – amino acids
- non – protein amino acids
- iso electric point
- glycine
- peptide bonds
- denaturation
- keratin, collagen
- Insulin, glucagon
- Enzymes
- Lipids
- emulsifier
- Lipids
- fact – soluble
- retinol
- Vitamin B2
- Vitamin B3
- Vitamin B5
- Vitamin B5
- Vitamin B12
- C
- Vitamin K
- nucleotides
- adenine, guanine
- 2’ – doxy – D -ribose, D-ribose
- nucleoside
- complementary base pairing
- 73 -94
- transcription
- mRNA
- DNA finger printing.
III. Match the column I and Column II using the code given below the column.
Question 1.
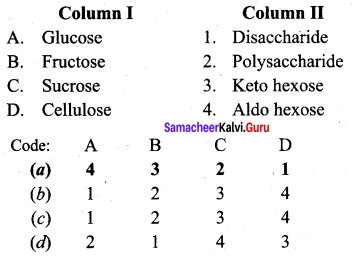
Answer:
(a) 4 3 2 1
Question 2.


Answer:
(b) 2 1 4 3
Question 3.
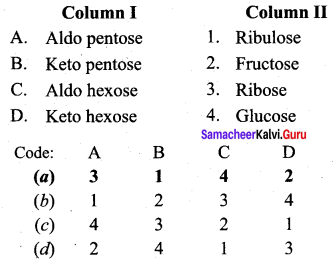
Answer:
(a) 3 1 4 2
Question 4.
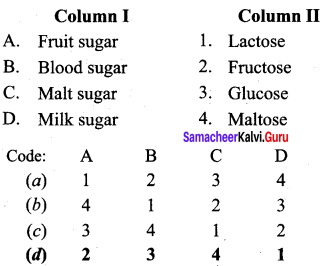
Answer:
(d) 2 3 4 1
Question 5.
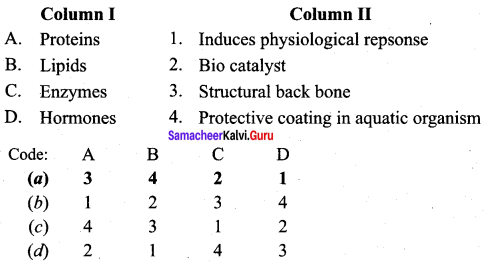
Answer:
(a) 3 4 2 1
Question 6.
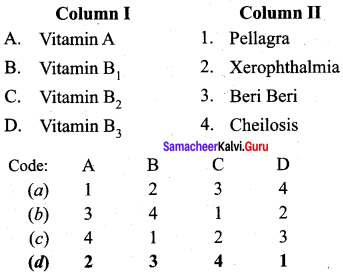
Answer:
(d) 2 3 4 1
Question 7.
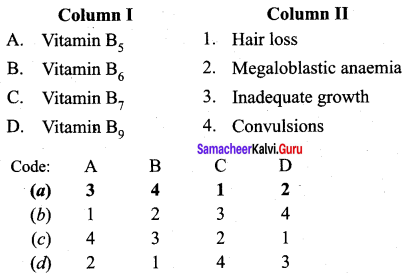
Answer:
(a) 3 4 1 2
Question 8.
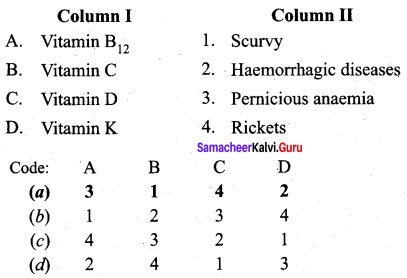
Answer:
(a) 3 1 4 2
Question 9.


Answer:
(a) 3 4 1 2
Question 10.
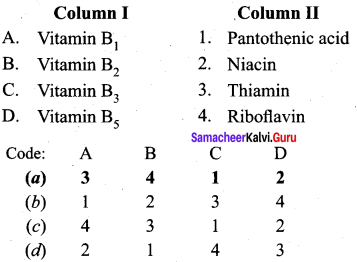
Answer:
(a) 3 4 1 2
Question 11.
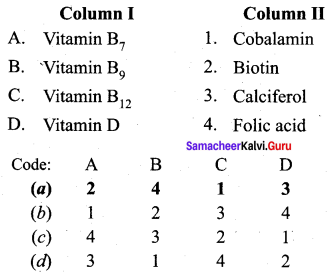
Answer:
(a) 2 4 1 3
Question 12.
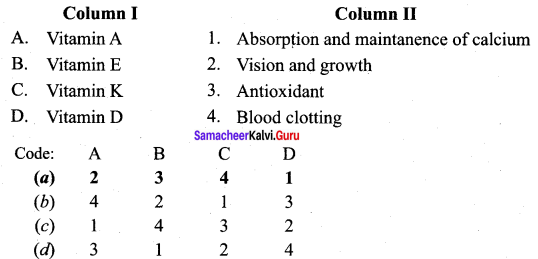
Answer:
(a) 2 3 4 1
IV. Assertion and reasons.
Question 1.
Assertlon(A): Almost all carbohydrates are optically active.
Reason (R): All carbohydrates have one or more chiral carbon atoms.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 2.
Assertion(A): Glucose is called blood sugar.
Reason (R): Human blood contains about 100 mg/di of glucose hence it is called blood sugar.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R ¡s the correct explanation of A.
Question 3.
Assertlon(A): Glucose is called aldohexose as well as dextrose.
Reason (R): Glucose contain an aldehyde group and it rotates the plane polarised light in the clockwise direction.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 4.
Assertion(A): Glucose contains an aldehyde group and it occupies one end of the carbon chain.
Reason (R): When glucose is oxidised by bromine water, it gets oxidised to gluconic acid confirms the position of aldehyde group.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 5.
Assertion(A): Glucose contains one primary alcohol group at the end of the carbon chain.
Reason (R): When glucose is oxidised by strong oxidising agent conc.HNO3 it gives glucaric acid proves the presence of- CH2OH group at one end of carbon chain in glucose.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 6.
Assertion(A): Glucose and mannose are epimers.
Reason (R): Sugars differing in configuration at an asymmetric centre are called epimers.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 7.
Assertion(A): Fructose is called levulose and keto hexose
Reason (R): Fructose contains a ketone group and fructose rotates the plane polarised light in anic1ockwise direction.
(a) Both A and R are wrong
(b) Both A and R are correct and R is the correct explanation of A.
(c) A is wrong but R is correct.
(d) A is correct hut R is wrong.
Answer:
(b) Both A and R are correct and R is the correct explanation of A.
Question 8.
Assertion(A): Sucrose is called invert sugar.
Reason (R): During hydrolysis of sucrose, the optical rotation of the reaction mixture changes from dextro to levo.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 9.
Assertion(A): Sucrose is a non reducing sugar.
Reason (R): in sucrose. C1 of α – D glucose and C2 of D-fructose are joined together by glycosidic bond. Both the carbonyl carbons are involved in glycosidic bonding.
(a) Both A and R are correct but R is not the correct explanation of A.
(b) Both A and R are correct and R the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are correct and R the correct explanation of A.
Question 10.
Assertion(A): A disaccharide lactose act as reducing sugar.
Reason (R): In lactose, β – D galactose and β – D glucose are linked by β – 1, 4 – glycosidic bond in which aldehyde group is not involved.
(a) A is correct but R is wrong.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) Both A and R are correct and R is the correct explanation of A.
(d) A is wrong but R is correct.
Answer:
(c) Both A and R are correct and R is the correct explanation of A.
Question 11.
Assertion(A): Lactose is referred to as milk sugar.
Reason (R): It is extracted from sprouted barley.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are worng.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(c) A is correct but R is wrong.
Question 12.
Assertion(A): Maltose, a disaccharide acts as a reducing sugar.
Reason (R): Maltose consists of two molecules of α – D glucose with linked by α, 1, 4 – glycosidic bond and one glucose has the carbonyl group.
(a) Both A and R are correct and R ¡s the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 13.
Assertion(A): Except glycine all other amino acids are optically active.
Reason (R): Glycine does not contain chira! carbon atom whereas in all other amino acids have chiral carbon atom.
(a) Both A and R are wrong.
(b) A is correct but R is wrong
(c) Both A and R are correct and R is the correct explanation of A.
(d) Both A and R are correct but R is not the correct explantion of A.
Answer:
(c) Both A and R are correct and R is the correct explanation of A.
Question 14.
Assertion(A): Enzymes have active sites and substrates, reactive sites on their surfaces respectively.
Reason (R): Active and reactive sites push the enzyme and substrate molecules away from each other.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 15.
Assertion(A): Enzymes are defined as biological proteins.
Reason (R): Chemically all enzymes are globular proteins.
(a) Both A and R are correct and R ¡s the correct explanation of A.
(b) Both A and R are wrong.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R ¡s the correct explanation of A.
Question 16.
Assertion(A): DNA and RNA molecules are found in the molecules of the cell.
Reason (R): On heating, enzymes do not lose their specific activity.
(a) Both A and R are correct and R explains A.
(b) Both A and R are wrong.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are wrong.
Question 17.
Assertion(A): Vitamin D can be stored in our body.
Reason (R): Vitamin D is a fat soluble vitamin.
(a) A is correct but R not explains A.
(b) Both A and R are correct and R explains A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are correct and R explains A.
Question 18.
Assertion(A): Glycine must be taken through diet.
Reason (R): It is an essential amino acid.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is wrong but R is correct.
(d) Both A and R are wrong.
Answer:
(d) Both A and R are wrong.
Question 19.
Assertion(A): In proteins, amino acids are linked through peptide bonds.
Reason (R): Peptide bonds are glycosidic (or) oxygen bridges.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explains A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(c) A is correct but R is wrong.
Question 20.
Assertion(A): Monosaccharides are held by glycocidic bonds.
Reason (R): Monosaccharides are macro molecules.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explains A.
(c) A is wrong but R is correct.
(d) Both A and R are wrong.
Answer:
(d) Both A and R are wrong.
V. Find the odd one out and give the reasons.
Question 1.
Glucose, fructose, galactose, mannose, sucrose.
Answer:
Sucrose, It is a disaccharide whereas other are monosaccharides.
Question 2.
Glucose, aldo hexose, dextrose, blood sugar, fruit sugar.
Answer:
Fruit sugar, It is the name of fructose, All others are indicating glucose only.
Question 3.
Fructose, fruit sugar, milk sugar, levulose, ketohexose.
Answer:
Milk sugar, It is the name of lactose, All others are indicating fructose only.
Question 4.
Mannose, sucrose, lactose, maltose, diastose.
Answer:
Mannose, It is a monosaccharide whereas otheres are disaccharide.
Question 5.
Keratm, glucose, mannose, starch, cellulose.
Answer:
Keratin, It is a protein whereas others are carbohydrates.
Question 6.
Keram, collagen, glycine, alanine, inulin, instilin.
Answer:
Inulin, It is a carbohydrates whereas others arc proteins.
Question 7.
Glycine, alanine, histidine, cultamine, proline, serine.
Answer:
Histidine. it is an essential amino acid whereas others are non essentîal amino acids.
Question 8.
Valine, phenyl alanine, histidine, lysine, alanine.
Answer:
Alanine, It is non essential amino acid whereas others are essential amino acids.
Question 9.
Invertase, maltase, zymase, maltose, lactase.
Answer:
Maltose, It is a carbohydrates whereas others are enzymes.
Question 10.
Vitamin A, Vitamin D, Vitamin C, Vitamin E, Vitamin K.
Answer:
Vitamin C is water soluble vitamins whereas others are fat soluble vitamins.
Samacheer Kalvi 12th Chemistry Biomolecules 2 Mark Questions and Answers
VI. Answer the following.
Question 1.
Define carbohydrates. Give example.
Answer:
- Carbohydrates are defined as polyhydroxy aldehydes (or) ketoses with a general formula Cn(H2O)n.
- They are considered as hydrates of carbon containing hydrogen and oxygen in the same ratio as in water.
- Example: Glucose, sucrose, cellulose.
Question 2.
Draw the structure of
- D – Glucose
- D – Fructose.
Answer:
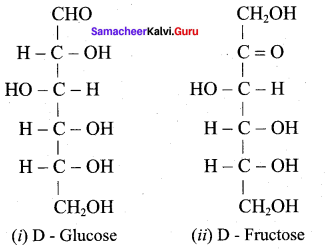
Question 3.
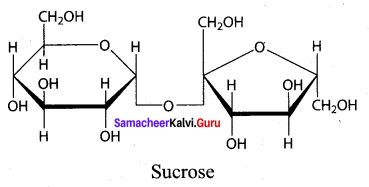
Draw the structure of sucrose
Answer:
Sucrose C12H22O11 is a disaccharide
Question 4.
Explain photosynthesis.
Answer:
Carbohydrates are synthesised by green leaves during photosynthesis, a complex process in which sunlight provides the energy to convert carbon dioxide and water into glucose and oxygen. Glucose is then converted into other carbohydrates and is consumed by animals.
![]()
Question 5.
Draw and explain the structure of glyceraldehyde.
Answer:
1. 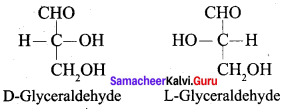
2. D – Glyceraldehyde and L – Glyceraldehyde are two enantiomers.
3. D (or) (+) is dextre rotatory and L (or) (-) is levo rotatory.
Question 6.
What is meant by dextro and levo rotatory?
Answer:
1. Fischer has devise a projection formula to reláte the two enantiomeric forms. Base on this, carbohydrates are named as D or L.
2. + and – sign indicates the dextro rotatory and levo rotatory respectively.
3. Dextre rotatory compound rotate the plane of plane polarised light in clockwise direction while the levo rotatory compounds rotate in anticlockwise direction.
4. Dextro rotatory compounds are represented as D (+) or L (+) and the levo rotatiory compounds as D (-) or L (-).
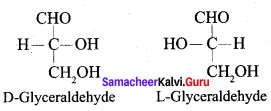
Question 7.
Give examples for the following.
- Aldotriose
- Ketotriose
- Aldotetrose
- Ketotetrose
Answer:
- Aldotriose – Glyceraldehyde
- Ketotriose – Dihydroxy acetone
- Aldotetrose – Erythrose
- Ketotetrose – Erythrulose
Question 8.
Give examples for the following.
- Aldo pentose
- Keto pentose
- Aldo hexose
- Ketohexose
Answer:
- Aldo pentose – Ribose
- Keto pentose – Ribulose
- Aldo hexose – Glucose
- Keto hexose – Fructose
Question 9.
Explain the action of Conc.HNO3 with fructose with equation.
Answer:
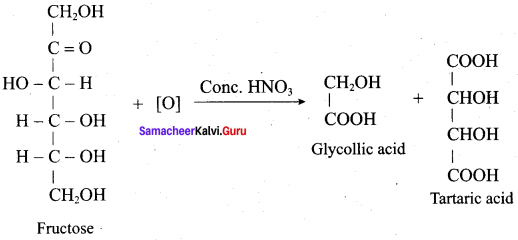
Question 10.
Write a note about glycogen?
Answer:
1. Glycogcn is the storage polysaccharide of animals. It is present in the liver and muscles of animals. Glycogen is called as animal starch.
2. Glycogen on hydrolysis gives glucose molecules. Structurally glycogen resembles amylo pectin with more branching. In glycogen the branching occurs every 8 – 14 glucose units opposed to 24 – 30 units in amylopectin. The excessive glucose in the body is stored in the form of glycogen.
Question 11.
What are amino acid? Give its structure.
Answer:
1. Amino acids are compounds which contain an amino group and a carboxylic group. Protein molecules are made up of α, β, γ – amino acids.
2. The protein molecules are mde up of a-amino acids which can be represented by the following general formula.
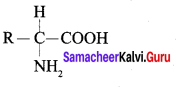
3. There are 20 amino acids commonly found in protein molecules.
Question 12.
Define iso electric point.
Answer:
At a specific pH value, the net charge of an amino acid in neutral is called iso electric point. At a pH value above the iso electric point the amino acid will be negatively charged and positively charged at pH values below the iso electric point.
Question 13.
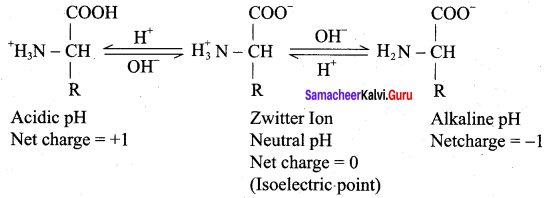
Whatis zwitter ion? Give its structure.
Answer:
1. At aqueous solution, the proton from cabroxyl group can be transferred to the amino group of an amino acid leaving these groups with opposite charges.
2. Despite having both positive and negative charges, this molecule is neutral and has amphotenc behaviour. These ions are called zwitter ions.
3. 
Question 14.
What are hormones? Mention their functions. Name some hormones.
Answer:
1. Hormones are organic compounds (eg. peptide or a steroid) that is secreted by endocrine glands. It is an inter cellular signalling molecule and induces a physiological response. Hormones maintain blood pressure, blood volume and electrolyte balance, embryogenesis, hunger, eating behaviour and digestion.
2. The major endogrine glands are the pituitary, pineal, thymus. thyroid, adrenal glands and pancreas. In addition men produces hormones in their testes and women produces hormones in their ovary.
Question 15.
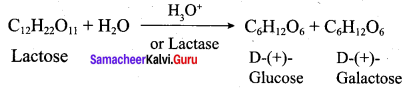
What are the expected products of hyrolysis of lactose?
Answer:
Since lactose is a disaccharide, therefore on hydrolysis it gives two molecules of monosaccharides, i.e., one molecule each of D – (+) – glucose and D – (+) – galactose
Question 16.
Glucose or sucrose are soluble in water by cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Answer:
Glucose and sucrose molecules contain many – OH groups and hence are capable of forming H – bond with water molecules. On the other hand, cyclohexane and benzene are hydrocarbon and hence they cannot form H-bond with water. Therefore, glucose and sucrose are soluble in water whereas cyclohexane and benzene are insoluble in water.
Question 17.
How do you explain the absence of aldehyde group in the pentaacetate of D – glucose?
Answer:
Since pentaacetate of D – glucose are not oxidised either by Tollen’s reagent or Fehling’s solution. This indicates the absence of aldehyde group.
Question 18.
Why cannot Vitamin C be stored in our body?
Answer:
Vitamin C is water soluble, therefore it is readily excreted in urine and hence cannot be stored in the body.
Question 19.
What do you understand by the term glycosidic linkage?
Answer:
In disaccharides or polysaccharides, monosaccharides are joined together by an oxide linkage formed by loss of a water molecule. Such a linkage between two monosaccaride units through oxygen atom is called glycosidic linkage.
Question 20.
What are essential and non-essential amino acids? Give two examples of each type.
Answer:
Essential amino acids:
Amino acids which cannot be synthesised in the body and must be obtained through regular diet are known as esential amino acids. Foe example: Valine, Leucine.
Non – essential amino acids:
The amino acids which can be synthesised in the body by itself are known as non – essential amino acids. For example: glycine, alanine.
Question 21.
What is the effect of denaturaion on the structure of proteins?
Answer:
During the denaturation process, 2° and 3° structure of proteins are destroyed by 1° structure remains intact. For example: curdling of milk.
Question 22.
How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer:
Vitamins are classified into two groups depending upon their solubility in water or fat.
1. Water soluble vitamins:
These include vitamins of ‘B’ group (except B12) and vitamin ‘C’.
2. Fat soluble vitamin:
These include vitamins A, D, E and K. They are stored in liver and adipose tissues. Vitamin ‘K’ is responsible for the coagulation of blood.
Question 23.
What are nucleic acids? Mention their two important functions.
Answer:
Nucleic acids are polymers of nucleotides containing a pentose sugar, heterocyclic base and a phosphate group. They help in synthesis of proteins. They are also responsible for the transfer of genetic characters from one generation to the next generation.
Question 24.
What is the difference between a nucleoside and a nucleotide?
Answer:
Nucleoside is formed by the condensation of a purine or pyrimidine base with pentose sugar at position 1. When nucleoside is linked to phosphoric acid at 5 position of sugar moiety, we get a nucleotide. Hence a nucleotide has three units – Phosphate group, pentose sugar and a base, whereas nucleoside has two units – pentose sugar and a base.
Question 25.
Write two main functions of carbohydrates in plants.
Answer:
Main functions of carbohydrates in plants:
- Carbohydrates are used as storage molecules as starch in plants.
- Cell wall of bacteria and plants is made up of cellulose.
Question 26.
Name two components of starch. How do they differ from each other structurally?
Answer:
The two components of starch are:
- Amylose
- Amylopectin
Amylose is a straight chain polymer of α – D – (+) glucose, while amylopectin is a branched chain polymer of α – D – glucose.
Question 27.
Name the four bases present in DNA. Which one of these is not present in RNA?
Answer:
DNA contains four bases, viz; adenine (A), guanine (G), cytosine (C), thymine (T). RNA also contain four bases, first three bases are same as in DNA but the fourth one is uracil(U).
Question 28.
Name two fat soluble vitamins, their sources and the diseases caused due to their deficiency in diet.
Answer:
Vitamins
- Vitamin A
- Vitamin D
Sources
- Fish, liver oil, carrot
- Sunlight, milk, egg yolk
Deficiency Diseases
- Night blindness
- Rickets and osteomalacia
Question 29.
Name two water soluble vitamins, their sources and the diseases caused due to their deficiency in diet.
Answer:
Vitamins
- Vitamin B<sub>1</sub>
- Vitamin C
Sources
- Yeast. milk, vegetables.
- Citrus fruits, amia and green leafy vegetables.
Deficiency Diseases
- Ben – ben
- Scurvy (bleeding gums)
Samacheer Kalvi 12th Chemistry Biomolecules 3 mark Question and Answers
VII. Answer the following questions.
Question 1.
Explain the methods of preparation of glucose.
Answer:
1. When sucrose is boiled with diI-H2SO4 in alcoholic solution, hydrolysis take place and glucose and fructose are formed.
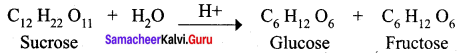
2. Glucose is produced commercially by the hydrolysis of starch with dilute HCL at high temperature and pressure.
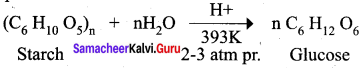
Question 2.
What happens when glucose reacts with
- Br2 / H2O
- Conc.HNO3
Answer:
1.
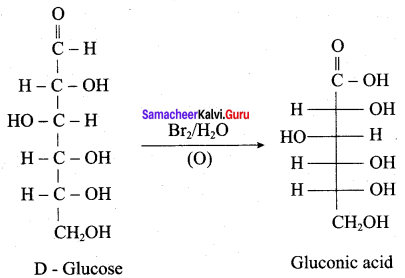
2.
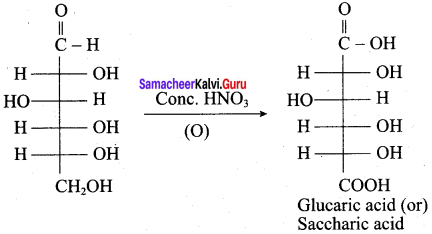
Question 3.
How will you prove the presence of aldehyde group in glucose?
Answer:
Glucose is oxidised to gluconic acid with ammoniacal silver nitrate (Tollen’s reagent) and alkaline copper sulphate (Fehling’s solution). Tollen’s reagent is reduced to metallic silver and Fehling’s solution to cuprous oxide (red precipitate). These reactions confirm the presence of an aldehye group in glucose.
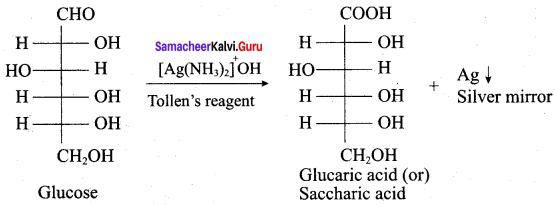
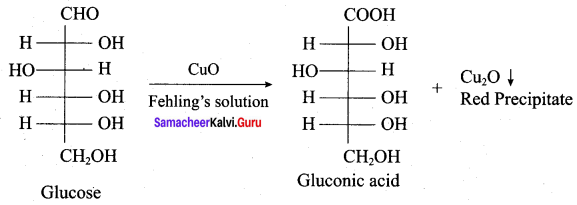
Question 4.
Define
- Epimers
- Epimerisation.
Answer:
1. Sugar differing at an asymmetric centre is known as epimers.
2. The process by which one epimer is converted into other is called epimerisation and it requires the enzyme epimersase.
3. Galactose is converted to glucose by this manner in our body.
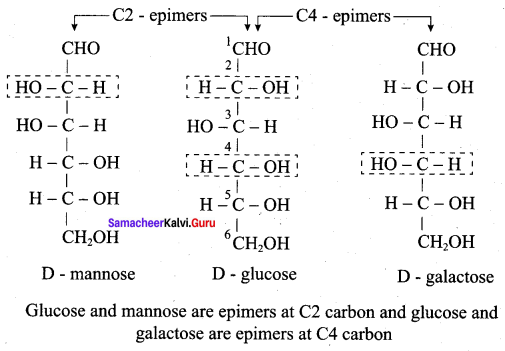
Question 5.
Explain the methods of preparation of fructose with equations.
Answer:
1. Fructose is obtained from sucrose by heating with dilute H2SO4 (or) with the enzyme invertase.
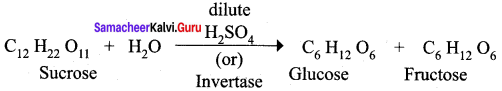
2. Fructose is prepared commercially by the hydrolysis of Inulin (a polysaccharide) in acidic medium
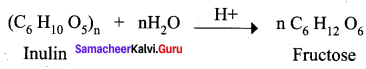
Question 6.
What happens when fructose is treated with sodium amalgam and water?
Answer:
When fructose is treated with sodium amalgam and water, partial reduction take place and the products formed are epimers of sorbitol and mannitol. New asymmetric carbon is formed at C – 2. This reaction confirms the presence of keto group in fructose.
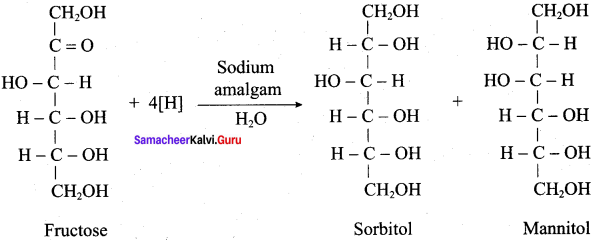
Question 7.
Explain about the cyclic structure of fructose?
Answer:
Fructose forms a five membered ring similar to furan. Hence it is called furanose form. When fructose is a component of a saccharide as in sucrose, it usually occurs in furanose form.
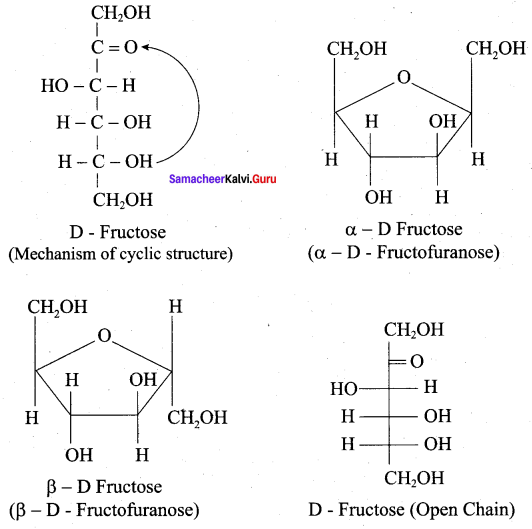
Question 8.
Explain about the structure, nature and properties of sucrose.
Answer:
1. Sucrose commonly known as table sugar is the most abundant disaccharide. It is obtained mainly from juice of sugar cane and sugar beets. Insects such as honey bees have the enzyme mvetase that catalyses the hydrolysis of sucrose into glucose and fructose mixture.
2. Honey is primarily a mixture of glucose, fructose and sucrose. On hydrolysis sucrose yields equal amount of glucose and fructose units.
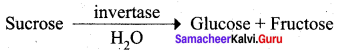
3. Sucrose (+66.6°) and glucose (52.5°) are dextrorotatory compounds while fructose is levo rotatory (- 92.4°)
4. During hydrolysis of sucrose the optical rotation of the reaction mixture changes from dextro to levo. Hence sucrose is also as invert sugar.
5. Structure:
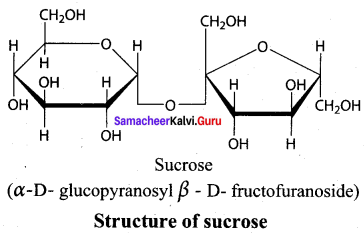
In sucrose C1 of α – D glucose is joined to C2 of D – Fructose. The glycosidic bond thus formed is called α, 1, 2 – glycosidic bond. Since both the carbonyl carbons are involved in the glycosidic bonding, sucrose is a non – reducing sugar.
Question 9.
Prove that sucrose is
- invert sugar
- non – reducing sugar.
Answer:
1. Sucrose is an invert sugar: Sucrose (+66.6°) and glucose (+52.5°) are dextro rotatory compounds while fructose is levo rotatory (- 92.4°). During hydrolysis of sucrose, the optical rotation of the reaction mixture changes from dextro to levo. Hence sucrose is also called invert sugar.
2. In sucrose, C1 of α – D glucose is joined to C2 of α – D fructose. The glycosidic bond thus formed is called α – 1, 2 – glycosidic bond. Since both the carbonyl carbons (reducing groups) are involved in the glycosidic bonding, sucrose is a non – reducing sugar.
Question 10.
Write a note about lactose
Answer:
1. Lactose is a disaccharide found in milk of mammals and hence it is referred to as milk sugar.
2. On hydrolysis, it yeilds galactose and glucose. The β – D galactose and β – D glucose are linked by β – 1, 4 – glycosidic bond. the aldehyde carbon is not involved in the glycosidic linkage. Hence it retains its reducing property and is called a reducing sugar.
3. 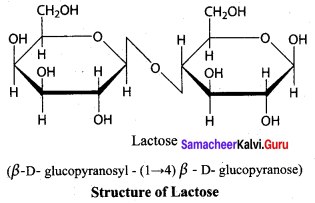
Question 11.
Lactose act as reducing sugar. Justify this statement.
Answer:
- Lactose is a disaccharide and contains one galactose unit and one glucose unit.
- In lactose, the β – D galactose and β – D glucose are linked by β – 1, 4 – glycosidic bond.
- The aldehyde carbon is not involved in the glycosidic bond hence it retains its reducing property and is called a reducing sugar.
Question 12.
Write about maltose with its structure.
Answer:
1. Maltose is extracted from malt and it is called malt sugar. Malt from sprouting barely is the major source of maltose. Maltose is produced during digestion of starch by the enzyme α – analyse.
2. 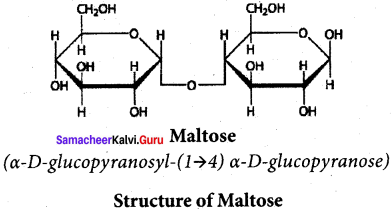
3. Maltose consists of two molecules of α – D glucose units linked by an α – 1, 4 – glycocidic bond between anomeric carbon ofone unit and C – 4 of the other unit. Since one of the glucose has the carbonyl group intact it act as a reducing sugar.
Question 13.
Sucrose and maltose are disaccharides but sucrose Is a non reducing sugar while maltose Is a reducing sugar. Give reason.
Answer:
1. Sucrose is a disaccharide that composed of α – D glucose and β – D fructose. In sucrose Cl of α – D glucose is joined to C2 of D – fructose. The glycosidic bond thus formed is called α – 1, 2- glycosidic bond. Since both the carbonyl carbons (reducing carbons) are involved in the glycosidic bonding, sucrose is a non-reducing sugar.
2. But maltose contains two molecules of x – D glucose units that are linked by an α – 1, 4 – glycosidic bond. Anomeric carbon of one unit and C – 4 of other unit are connected together. Smce one of the glucose bas the carbonyl group intact it acts as reducing sugar.
Question 14.
Give brief account of nature and structure of cellulose.
Answer:
1. Cellulose is the major constituent of plant cell walls. Cotton is almost pure cellulose. On hydrolysis, cellulose yields D – glucose molecules
2. Cellulose is a straight chain polysaccharide. The glucose molecules are linked by β (1, 4 -) glycoside bond.
3. Structure of cellulose.
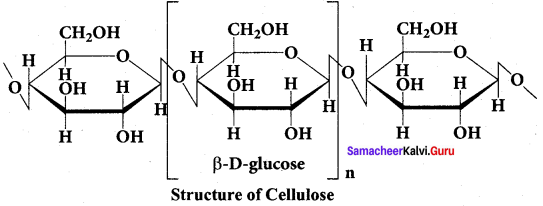
Question 15.
What are the uses of cellulose?
Answer:
- Cellulose is used extensively in manufacturing paper, cellulose fibres and rayon explosive.
- Gun cotton – nitrated ester of cellulose an explosive is prepared from cellulose.
- Cellulose act as food for animals
Question 16.
Human cannot use cellulose as food – Why?
Answer:
Human cannot use cellulose as food because our digestive systems do not contain the necessary enzymes such as glycosidases (or) cellulases that can hydrolyse the cellulose. But animals contain cellulose enzyme in their digestive system and they can digest cellulose. So cellulose can used as food for animals but not for human.
Question 17.
What are the major classification of proteins? Give example?
Answer:
1. Proteins are classified based on their structure into two major types. They are fibrous protein and glubular protein.
2. Fibrous proteins are linear molecules similar to fibres. They are generally insoluble in water and are held together by disulphide bridges and weak inter molecular hydrogen bonds. These proteins often used as structural proteins. Example, Keratin, Collagen.
3. Globular proteins have an overall spherical shape. The poly peptide chain is folded into a spherical shape. These proteins are usually soluble in water and have many functions including catalysis.
Question 18.
Explain the mechanism of enzyme action?
Answer:
1. Enzymes are bio catalysts that catalyse a specific bio chemical reaction. They generally activate the reaction by reducing the activation energy by stabilising the transition state.
2. In a typical reaction, enzyme E binds with the substrate molecule leversity to produce an enzyme – sybstate complex. During this stage the substrate is converted into product and
3. 
Question 19.
Explain about the nature, classification and properties of lipids (or) write a note about lipids.
Answer:
1. Lipids are organic molecules that are soluble in organic solvents such as chloroform and methanol and are insoluble in water. Lipids means fat. They are the principal component of cell membrane including cell walls.
2. Lipids act as energy source for living systems. Fat provide 2 – 3 fold higher energy compared to carbohydrates or proteins.
3. Based on their structure, lipids can be classified as simple lipids, compound lipids and derived lipids.
4. simple lipids can be further classified into fats, which are esters of long chain fatty acids fats, with glycerol (triglycerides) and waxes which are esters of fatty acid with long chain monohydric alcohols (Bees wax)
5. Compound lipids are the esters of simple fatty acid with glycerol which contain additional group. Based on the groups attached, they are further classified into phospholipids, glycolipids and lipproteins. Phospho lipids contain a phospho ester linkage while the glycolipids contain a sugar molecule attached. The iipo proteins are complexes of lipid with proteins.
Question 20.
Write the chemical name, sources and deficient disease of the following.
- Vitamin D
- Vitamin E
- Vitamin K
Answer:
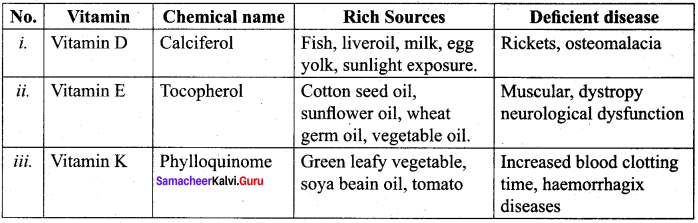
Question 21.
What are the biological functions of nucleic acids?
Answer:
- Energy carriers (ATP)
- Components of enzyme cofactors. Example – Co enzyme A, NAD, FAD
- Chemical messengers. Example – Cyclic AMP, CAMP
Question 22.
What happens when D – glucose is treated with the following reagents?
- HI
- Bromine water
- HNO3
Answer:
1. 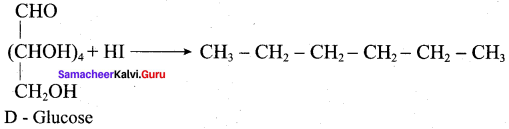
2.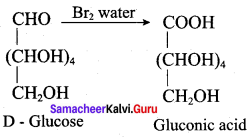
3. 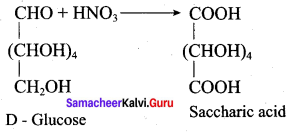
Question 23.
Define the following as related to proteins.
- Peptide linkage
- Primary structure
- Denaturation
Answer:
1. Peptide linkage:
Amino acids are bifunctional molecules with NH2 group at its one end and COOH at the other. Therefore, the COOH of one molecule and NH2 of another molecule can interact with elimination of a H,O molecule to form an amide like linkage called peptide bond or peptide linkage.
2. Primary structure:
The sequence in which amino acids are linked together in a polypeptide chain forms the primary structure.
3. Denaturation:
The process by which secondary and tertiary structure of proteins get disturbed by change of pH or temperature, so that they are not able to perform their functions, is called denaturation of proteins.
Question 24.
Difference between Globular and fibrous proteins.
Answer:
Globular proteins
- They form α – helix structure
- They are soluble in water.
- They are cross lined condensation polymers of acidic and basic amino acids.
Fibrous Proteins
- They have – plcated structure.
- They are insoluble in water.
- They are linear condensation polymeric proteins.
Question 25.
Explain what is meant by
- a peptide linkage
- a glycosidic linkage.
Answer:
1. Peptide linkage:
Polymers of x – amino acids are connected to each other by peptide bond or peptide linkage. Chemically peptide linkage is an amide formed between – COOH group and NH2 group.
2. Glycosidic linkage:
The two monosaccharide units are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two mono saecharide units through oxygen atom is called glycosidic linkage.
Question 26.
What are essential and non-essential amino acids? Give one example of each type.
Answer:
1. Essential amino acids:
Amino acids which are not synthesised by the human body are called essential amino acids. Example – Valine, Leucinc.
2. Non – essential amino acids:
Amino acids which are synthesised by human body are called non – essential amino acids. Example – Glycine, Aspartic acid, etc.
Question 27.
Mention the type of linkage responsible for the formation of the following.
- Primary structure of proteins
- Cross linking of polypeptide chains.
- α – helix formation
- β – sheet structure.
Answer:
Type of Structure
- Primary structure of proteins
- Cross linking of polypeptide chains
- α – helix
- β – sheet structure
Type of linkages
- Peptide bond or peptide linkage
- Polypeptide linkage
- Hydrogen bond
- Intermolecular hydrogen bond
Question 28.
Name the chemical components which constitute nucleotides. Write any two functions of nucleotides in a cell.
Answer:
Nucleotides are made up of a heterocyclic base containing nitrogen, a five carbon atom – moeity and a phosphate group.
Example:
- AMP (adenosine monophosphate)
- ADP (adenosine diphosphate) and
- ATP (adenosine triphospate)
Functions:
- Act as energy carriers
- They synthesise proteins
Question 28.
Name the main disease caused due to lack of vita min and its source in each of the following. A, B6 and E.
Answer:

Question 29.
Define the following and give one example of each.
- Isoelectric point
- Mutarotation
- Enzymes
Answer:
1. Isoelectric point:
It is the pH at which +ve and – ve charges on zwitter ion are equal. Example amino acid exists as zwitter ion at pH = 5.5 to 6.3.
2. Mutarotation:
It is a spontaneous change in optical rotation when an optically active substance is dissolved in water. Example α – glucose, when dissolved in water, then its optical rotation changes from 1110 to 52.5°.
3. Enzymes:
Enzymes are biocatalysts which speeds up the reactions in biosystems. They are highly specific and selective in their action. Chemically all enzymes are proteins.
Question 30.
What is denaturation and renaturation of proteins? Give reason: Amylose present in the saliva becomes inactive in the stomach.
Answer:
1. The process of disruption of 2° and 3° structure of proteins without changing its primary structure is called denaturation.
2. In stomach the pH is acidic, therefore amylose becomes inactive. That is why digestion of carbohydrates does not take place in stomach.
Question 31.
Define the following terms.
- Nucleotide
- Anomers
- Essential amino acids
Answer:
1. Nucleotide:
It is the monomer unit of DNA which is formed by a nitrogenous base, deoxyribose sugar and phosphoric acid.
2. Anomers:
Anomers are cyclic monosaccharides which are differing from each other in the configuration of C – 1 if it is an aldose or in the configuration at C – 2 if it is a ketose.
3. Essential amino acids:
The amino acids cannot be synthesised by the body and are essential for the body.
Question 32.
Which one of the following is disaccharide.
- Starch, Maltose, Fructose, Glucose.
- Write the name of vitamin whose deficiency causes bone deformities in children.
Answer:
- Maltose
- Vitamin D
Quesiton 33.
Write the major classes in which the carbohydrates are divided depending upon whether thee undergo hydrolysis and if so, the number of products formed.
Answer:
On the basis of hydrolysis, carbohydrates are divided into three major classes:
1. Monosaccharides. These cannot be hydrolysed into simpler molecules. These are further classified as aldoses, and ketoses.
2. Oligosaccharides. These carbohydrates on hydrolysis give 2-10 units of mono-saccharides. For example – Sucrose.
3. Polysaccharides. These are high molecular mass carbohydrates which give many molecules of monosaccharides on hydrolysis. Form example: Cellulose, starch.
Question 34.
- What changes occur in the nature of egg proteins on boiling?
- Name the type of bonding which stabilises the a-helix structure in proteins.
Answer:
1. On boiling, protein of egg gets denatured. Thus, due to coagulation water get absorbed.
2. Hydrogen bonding between

Question 35.
Answer the following questions briefly.
- What are reducing sugars?
- What is meant by denatu ration of a protein?
- How is oxygen replenished in our atmosphere?
Answer:
1. Reducing sugar:
All those carbohydrates which reduce Fehling’s solution and Tollens’ reagent are referred to as reducing sugars. All monosaccharides whether aldose or ketose are reducing sugars.
2. Denaturation of a protein:
When 2° and 3° structure of a protein is destroyed due to the physical changes like temperature, change in pH, it is called denaturation of a protein.
Example: Coagulation of egg white on boiling.
3. We take oxygen from atmosphere and release CO2. Plants take up CO2 and H2O from the atmosphere to prepare their food in the presence of sunlight and release O2 thus O2 is replenished in atmosphere.
Samacheer Kalvi 12th Chemistry Biomolecules 5 mark Questions Answers
VIII.Answer the following questions.
Question 1.
How would you prove the structure of glucose? (OR) Elucidate the structure of glucose.
Answer:
1. Elemental analysis and molecular weight determination show that the molecular formula of glucose is C6H12O6.
2. On reduction with Conc.HI and red P at 373K, glucose gives a mixture of n – hexane and 2 – iodohexane indicating that the six carbon atoms are bonded linearly.
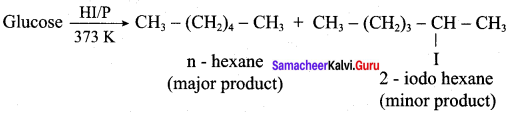
3. Glucose reacts with hydroxylamine to form oxime and with HCN to form cyanohydrin. These reactions indicate the presence of carbonyl group in glucose.
4. Glucose gets oxidised to gluconic acid with bromine water with bromine water suggests that the carbonyl group is an aldchyde group and it occupies one end of the carbon chain. When glucose is oxidised by conc.HNO3, glucaric acid is formed and it suggest that the other end Id occupied by a primary alcoholi.
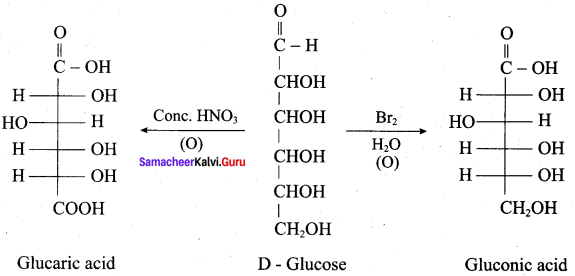
5. Glucose is oxidised to gluconic acid with ammoniacal silver nitrate (Toiles reagent) and alkaline copper sulphate (Fehling’s solution). These reactions further confirm the presence of an aldehyde group.
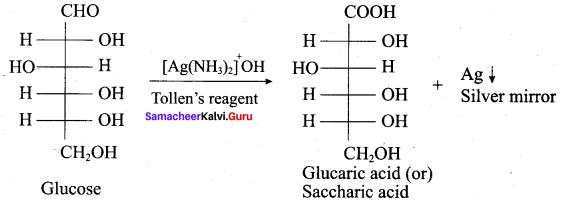
6. Glucose forms penta acetate with acetic anhydride suggesting the presence of five alcohol groups.
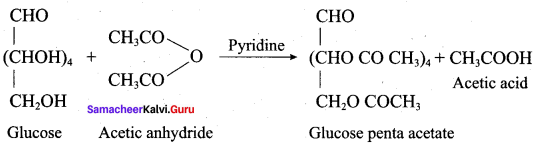
7. Glucose is a stable compound and does not undergo dehydration easily. It indicates that not more than one hydroxyl group is bonded to a single carbon atom. Thus the five hydroxyl groups are attached to five different carbon atoms and the sixth carbon is an aldehyde group.
8. The exact special arrangement of – OH groups was given by Emil Fischer as follows.
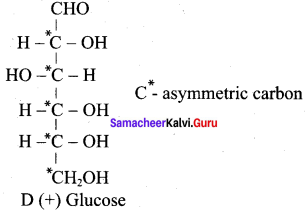
9. D (+) glucose has D configuration and it is dextro rotatoly.
Question 2.
Explain about the cyclic structure of Glucose.
Answer:
1. Fischer identified that the open chain Penta hydroxyl aldehyde structure of glucose that he proposed did not completely explain its chemical behaviour.
2. Unlike simple aldehyde, glucose did not form crystalline hisuiphite compound with sodium bisuiphite. Glucose does not give Schifis test and pent.a acetate derivative of glucose was not oxidised by Tollen’s reagent. This behaviour could not be explained by open chain structure.
3.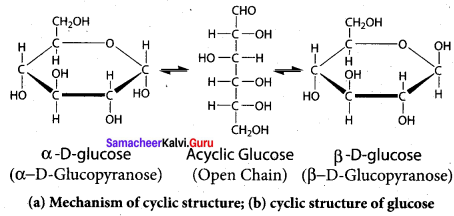
4. In order to explain these it was proposed that one of the hydroxyl group reacts with aldehyde group to form a cyclic structure (hemiacetal form). This also results in the conversation of the achiral aldehyde carbon into a chiral one leading the possibility of two isomers. These two isomers differ only in the configuration of C1 carbon. These isomers are called anomers.
5. The two anomeric forms of glucose are called – and β – forms. This cyclic structure of glucose is similar to pyran, a cyclic compound with 5 carbon and one oxygen atom, and hence is called pyranose form.
6. The specific rotation of pure α – and 3 – (D) glucose are 112° & 18.7° respectively. However, when pure form any one of these sugars dissolved in water, slow interconversion of – D glucose and β – D glucose via open chain form until equilibrium is established giving constant specific rotation + 53°. This phenomenon is called mutarotation.
Question 3.
Explain about the structure of Fructose. (OR) Elucidate the structure of Fructose.
Answer:
1. Elemental analysis and molecular weight determination of fructose show that it has the molecular formula C6H12O6.
2. Fructose on reduction with Hl and red phosphoms gives a mixture of n – hexane (major product) and 2 – iodohexane (minor product). This reaction indicates that the six carbon atoms in fructose are in a straight chain.

3. Fructose reacts with NH2OH and HCN. It shows the presence of carbonyl groups in the molecules of fructose.
4. Fructose reacts with acetic anhydride in the presence of pyridine to form penta acetate. This reaction indicates the presence of’ five hydroxyl groups in a fructose molecule.
5. Fructose is not oxidized by bromine water. This rules out the possibility of presence of an aldehyde (-CHO) group.
6. Partial reduction of fructose with sodium amalgam and water produces mixtures of sorbitol and mannitol which are epimers at second carbon. New asymmetric carbon is formed at C – 2. This confirms the presence of keto group.
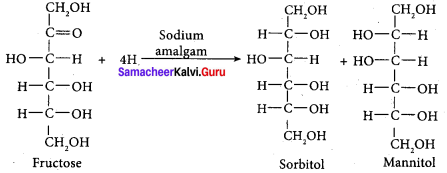
7. On oxidation with nitric acid, it gives glycolic acid and tartane acids which contain smaller number of carbon atoms than in fructose. This shows that a keto group is present in C – 2. It also shows the presence of 10 alcoholic groups at C- 1 and C- 6. From the above reaction the structure of fructose is
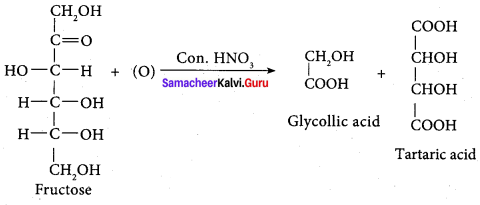
This shows that a keto group is present in C – 2. It also shows the lpresence of 10 alcoholic groups at C – 1 and C – 6. From the above reaction the structure of fructose is
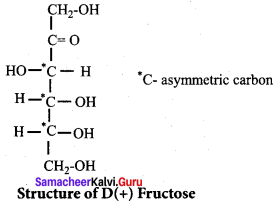
Question 4.
Describe about the structure, nature and properties of starch.
Answer:
- Starch is used for energy storage in plants. Potatoes, corn, wheat and rice are the rich sources of starch.
- It is a polymer of glucose in which glucose molecules are lined by α (1,4) glycosidic bonds.
- Starch can be separated into two fractions namely, water soluble amylose and water insoluble amylo pectin. Starch contain about 20% of amylase and about 80% amylopectin.
- Amylose is composed of unbranched chains upto 4000 α – D glucose molecules joined by α (1,4) glycosidic bonds.
- At branch points, new chains of 24 to 30 glucose molecules are linked by α (1,6) glycosidic bonds with iodine solution amylose gives blue colour while amylo pectin gives a purple colour.
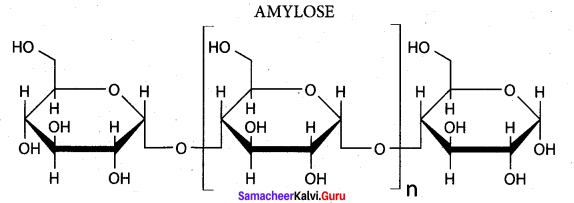
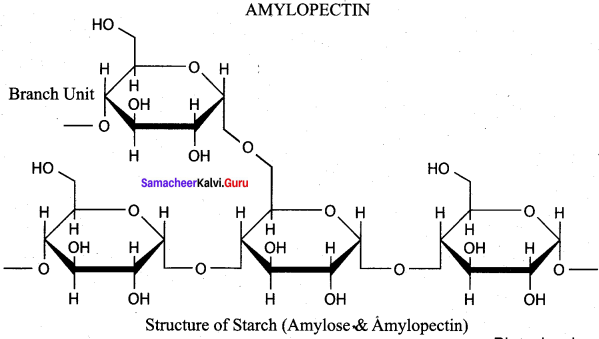
Question 5.
Explain about the structure of proteins.
Answer:
1. Proteins are polymers of amino acids. Their three dimensional structure depends mainly on the sequence of amino acids. The protein structure can be described at four hierarchal levels called primary, secondary, tertiary and quaternary structures.
Primary structure of proteins:
Proteins are polypeptide chains made up of amino acids connected through peptide bonds. The relative arrangement of the amino acids in the polypeptide chain is called the primary structure of the protein.

Secondary structure of proteins:
The amino acids in the polypeptide chain forms highly, regular shapes through the hydrogen bond between the carbonyl oxygen and the
neighbouring amine hydrogen of the main chain, α – Helix and β – strands or sheets are two most common substructures formed by proteins.
α – Helix:
In the α – helix sub – structure, the amino acids are arranged in a righthanded helical (spiral) structure and are stabilised by the hydrogen bond. The side chains of the residues protrude outside of the helix. Each turn of an α – helix contains about 3.6 residues and is about 5.4 A long.
β – Strands:
β – Strands are extended peptide chain rather than coiled. The hydrogen bond occur between main chain carbonyl group one such strand and the amino group of the adjacent strand resulting in the formation of a sheet like structure. This arrangement is called β – sheets.
Tertiary structure:
The secondary structure elements (α – helix & β – sheets) further folds to form a three dimensional arrangement. This tertiary structure of proteins are stabilised by the interactions between the side chains of the amino acids. These interactions include the disulphide bridges between cysteine residues, electrostatic, hydrophobic, hydrogen bonds and van der Waals interactions.
Quaternary Structure:
The oxygen transporting protein, haemoglobin contains four polypeptide chains while DNA polymerase enzyme that make copies of DNA, has ten polypeptide chains. In these proteins the individual polypeptide chains interacts with each other to form the multimeric structure which known as quaternary structure. The interactions that stabilises the tertiary structures also stabilises the quaternary structures.
Question 6.
What are the biological importance of proteins?
Answer:
Proteins are the functional units of living things play vital role in all biological processes
- All biochemical reactions occur in the living systems are catalysed by the catalytic proteins called enzymes.
- Proteins such as keratin, collagen acts as structural back bones.
- Proteins are used for transporting molecules (Haemogiobin), organelles (Kinesins) in the cell and control the movement of molecules in and out of the cells (Transporters).
- Antibodies help the body to fight various diseases.
- Proteins are used as messengers to coordinate many functions. Insulin & glucagon controls the glucose level in the blood.
- Proteins act as receptors that detect presence of certain signal molecules and activate the proper response.
- Proteins are also used to store metals such as iron (Femtin) etc.
Question 7.
Write the chemical name, source and deficient disease of the following
- Vitamin A
- Vitamin B1
- Vitamin B2
- Vitamin B3
- Vitamin B5
Answer:
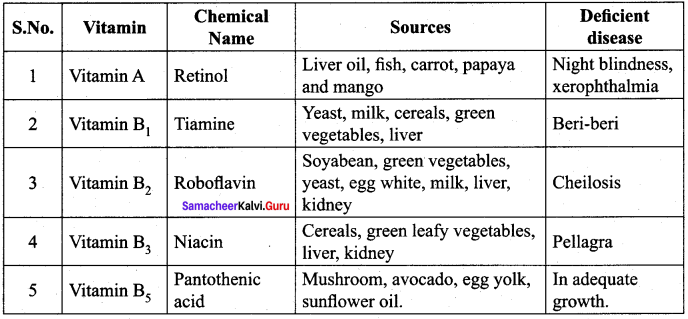
Question 8.
Write the chemical name, source and deficient disease of the following
- Vitamin B6
- Vitamin B7
- Vitamin B9
- Vitamin B12
- Vitamin C
Answer:
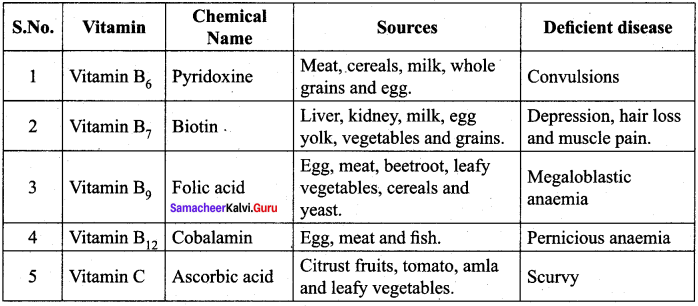
Question 9.
Explain about the composition and structure of nucleic acids.
Answer:
1. Nucleic acids are biopolymers of nucleotides. Controlled hydrolysis of DNA and RNA yields three components namely a nitrogeneous base, a pentose sugar and phosphate group.
2. Nitrogen base.
(a) These are nitrogen containing organic compounds which are derivatives of two parent compounds, pyrimidine and purine.
(b) Both DNA and RNA have two major purine bases, adenine (A) and guanine (G). In both DNA and RNA, one of the pyrimidines is cytosinc (C), but the second pyrimidine is thymine (T) ¡n DNA and uracil (U) in RNA.
3. Pentose Sugar.
Nucleic acids have two types ofpentoses. The recurring deoxyribonucleotide units of DNA contain 2’ – dcoxy – D – ribose and the ribonucleotide units of RNA contain D – ribose. In nucleotides, both types of pentoses are in their – furanose form.
4. Phosphate group.
Phosphoric acid forms phosphor diester bond between nucleotides. Based on the number of phosphate group present in the nucleotides, they are classified mono nucleotide, dinucleotide and trinucleotide.
5. The molecule without the phosphate group is called a nucleoside. A nucleotide is derived from a nucleoside by the addition of a molecule of phosphoric acid.
6. Sugar + Base → Nucleoside
Nucleoside + Phosphate → Nucleotidc
Nucleotide → Polynucleotide (Nucleic Acid)
Question 10.
Describe about the double strand helix structure of DNA.
Answer:
1. Watson and Crick postulated a 3 – dimensional model of RNA structure which consisted of two antiparallel helical DNA chains wound around the same axis to form a right handed double helix.
2. Th e hydrophilic backbones of alternating deoxyribose and phosphate groups are on the outside of the double helix, facing the surrounding water. Th e purine and pyrimidine bases of both strands are stacked inside the double helix,
with their hydrophobic and ring structures very close together and perpendicular to the long axis, thereby reducing the repulsions between the charged phosphate groups. The offset pairing of the two strands creates a major groove and minor groove on the surface of the duplex.
3. The model revealed that there are 10.5 pairs (36A°) per turn of the helix and 3.4A° between the stacked bases. They also found that each base is hydrogen bonded to a base in opposite strand to form a planar base pair.
4. Two hydrogen bonds are formed between adenine and thymine and three hydrogen bonds are formed between guanine and cytosine. Other pairing tends to destablize the double helical structure. This specific association of the two chains of the double helix is known as complementary base pairing.
5. The DNA double or duplex is held together by two forces.
- Hydrogen bonding between complementary base pairs.
- Base – stacking interactions.
The complementary between the DNA strands is attributable to the hydrogen bonding between base pairs but the base stacking interactions are largely non-specific, make the major contribution to the stability of the double helix.
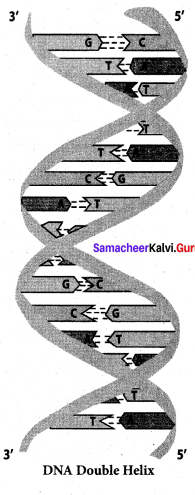
Question 11.
Explain about the types of RNA molecules.
Answer:
1. Ribonucleic acids are similar to DNA. Cells contain upto eight times high quantity of RNA than DNA. RNA is found in large amount in the cytoplasm and a lesser amount in the nucleus.
2. RNA molecules are classified according to their structure and function into three major types.
- Ribosomal RNA (r – RNA)
- Messenger RNA (m – RNA)
- Transfer RNA (t – RNA)
3. r – RNA:
r – RNA is mainly found in cytoplasm and in ribosomes, which contain 60% RNA and 40% protein. Ribosomes are the sites at which protein synthesis takes place.
4. t – RNA:
t – RNA molecules have lowest molecular weight of all nucleic acids. They consist of 73 – 94 nucleotides in a single chain. The function of t – RNA is to carry amino acids to the sites of protein synthesis on ribosomes.
5. m – RNA:
m – RNA is present in small quantity and very short lived. They are single stranded and their synthesis take place on DNA. The synthesis m-RNA from DNA strand is called transcription. m – RNA carries genetic information from DNA to the ribosomes for protein synthesis.
Question 12.
Explain about DNA finger printing process.
Answer:
1. DNA finger printing is one of the most accurate methods for placing an individual at the scene of a crime has been a finger print.
2. The DNA finger print is unique for every person and can be extracted from traces of sample from blood, saliva, hair etc. By using this method, we can detect the individual specific variation in human DNA.
3. In this method, the extracted DNA is cut at specific points of varying lengths in the formation of DNA fragments of varying lengths which were analysed by technique called gel electrophoresis. This method separates the fragments based on their size. The gel containing the DNA fragments are then transferred to a nylon sheet using a technique called blotting. Then the fragments will undergo autoradiography in which they were exposed to DNA probes.
4. A piece of X-ray film was then exposed to the fragments, and a dark mark was produced at any point where a radioactive probe had become attached. The resultant pattern of marks could then be compared with other samples.
5. DNA finger printing is based on slight sequence differences between individuals. These methods are providing decisive in court cases world wide.
Common Errors
- Carbohydrates & Enzyme names are different.
- Peptide bond and amide bond are different.
- Vitamins: Solubility based classification
- Main source of vitamin
Rectifications
1. Carbohydrates name should end as – ose.
Eg. Diatose, maltose
Enzyme name should end as – ase.
Eg. Diastase, maltase
2. 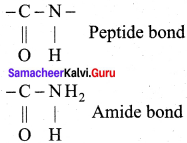
3. Vitamin A, D, E, K – fat soluble. Vitamin C, B complex are water soluble.
4. Milk:
Vitamin A, B1, B2, B6, B7, D (6 Vitamins)
Green Vegetables:
B1, B3, B7, K (4 vitamins)
Liver:
B1, B2, B3, B7
We hope the Class 12th Samacheer Kalvi Chemistry Solutions Chapter 14 Biomolecules Book Solutions Answers Guide Solutions provided here help the students to get the best knowledge. Also, students can use our Samacheer Kalvi Class 12th Chemistry Solutions Chapter 14 Biomolecules Questions and Answers for better practice. You can contact us at any moment by giving comments in the comment section. Keep checking updates by bookmarking our website.