Tamilnadu State Board Solutions for Class 12th Chemistry Solutions Chapter 2 p-Block Elements – I Questions and Answers will help you to improve the complete subject knowledge. Every student must look at the single concept included in Samacheer Kalvi 12th Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions Answers Guide Solutions PDF. All the concepts are explained clearly with examples and pictures.
Students can easily avoid the struggle to get the best book for Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions Answers Guide learning. You have to go through Chapterwise Samacheer Kalvi Class 12th Textbook Solutions for Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions for better practice.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 2 p-Block Elements – I
Students who wish to have the strong basics of Chemistry Solutions Chapter 2 p-Block Elements – I can use Tamilnadu State Board Class 12th Chemistry Solutions Chapter 2 p-Block Elements – I Questions and Answers Guide pdf. Enhance your knowledge by referring to the Samacheer Kalvi Solutions pdf. We provided a free pdf of Tamilnadu State Board Class 12th Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions Answers Guide material and textbook for students. Check it out now and start preparing for the exam immediately. Score maximum marks in the exam by referring to Samacheer Kalvi Class 12th Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions Answers Guide Solutions Pdf.
Samacheer Kalvi 12th Chemistry p-Block Elements – I TextBook Evalution
I. Choose the correct answer:
12th Chemistry Chapter 2 Book Back Answers Question 1.
An aqueous solution of borax is …………
(a) neutral
(b) acidic
(c) basic
(d) amphoteric
Answer:
(c) basic.
![]()
12th Chemistry Lesson 2 Book Back Answers Question 2.
Boric acid is an acid because its molecule …………
(a) contains replaceable H+ ion
(b) gives up a proton
(c) combines with proton to form water molecule
(d) accepts OH– from water, releasing proton.
Answer:
(d) accepts OH– from water, releasing proton
Hint: B(OH)3 + H2O \(\rightleftharpoons\) [B(OH)4]–+ H+
Samacheer Kalvi Guru 12th Chemistry Question 3.
Which among the following is not a borane?
(a) B2H6
(b) B3H6
(C) B4H10
(d) none of these
Answer:
(a) B2H6
Hint:
- Nido borane – BnH4+n
- aracno borane – BnH6+n , B3H6 is not a borane
Chapter 2 Chemistry Class 12 Notes Question 4.
Which of the following metals has the largest abundance in the earth’s crust?
(a) Aluminium
(b) calcium
(c) Magnesium
(d) Sodium
Answer:
(a) Aluminium
12th Chemistry Samacheer Kalvi Question 5.
In diborane, the number of electrons that accounts for banana bonds is …………
(a) six
(b) two
(c) four
(d) three
Answer:
(c) four
Hint: There are two 3c – 2e– bonds i.e., the bonding in the bridges account for 4 electrons.
12th Chemistry Evaluate Yourself Answers Samacheer Question 6.
The element that does not show catenation among the following p-block elements is …………
(a) Carbon
(b) silicon
(c) Lead
(d) germanium
Answer:
(c) Lead
Samacheer Kalvi 12th Chemistry Question 7.
Carbon atoms in fullerene with formula C60 have …………
(a) sp3 hybridised
(b) sp hybridised
(c) sp2 hybridised
(d) partially sp2 and partially sp3 hybridised
Answer:
(c) sp2 hybridised
Samacheer Kalvi Class 12 Chemistry Solutions Question 8.
Oxidation state of carbon in its hydrides …………
(a) +4
(b) -4
(c) +3
(d) +2
Answer:
(a) +4
Hint: CH4+in which the oxidation state of carbon is 4.
Samacheerkalvi.Guru 12th Chemistry Question 9.
The basic structural unit of silicates is …………
(a) (SiO3)2-
(b) (SiO4)2-
(c) (SiO)–
(d) (SiO4)4-
Answer:
(d) (SiO4)4-
Samacheer Kalvi 12th Chemistry Solutions Question 10.
The repeating unit in silicone is …………
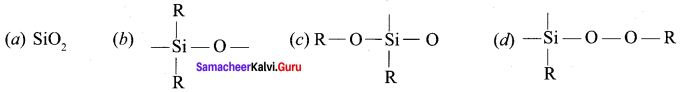
Answer:
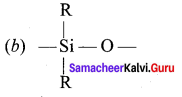
12 Chemistry Samacheer Kalvi Question 11.
Which of these is not a monomer for a high molecular mass silicone polymer?
(a) Me3SiCl
(b) PhSiCl3
(c) MeSiCl3
(d) Me3SiCl3
Answer:
(a) Me3SiCl
Samacheer 12 Chemistry Solutions Question 12.
Which of the following is not sp2 hybridised?
(a) Graphite
(b) graphene
(c) Fullerene
(d) dry ice
Answer:
(a) dry ice
Hint: dry ice – solid CO2 in which carbon is in sp hybridized state
Chemistry Class 12 Samacheer Kalvi Question 13.
The geometry at which carbon atom in diamond are bonded to each other is …………
(a) Tetrahedral
(b) hexagonal
(c) Octahedral
(d) none of these
Answer:
(a) Tetrahedral
Samacheer Kalvi 12th Chemistry Guide Question 14.
Which of the following statements is not correct?
(a) Beryl is a cyclic silicate
(b) Mg2SiO4 is an orthosilicate
(c) SiO44- is the basic structural unit of silicates
(d) Feldspar is not aluminosilicate
Answer:
(d) Feldspar is a three dimensional silicate
Question 15.
AlF3 is soluble in HF only in the presence of KF. It is due to the formation of ………… [NEET]
(a) K3[AlF3H3]
(b) K3[AlF6]
(C) AlH3
(d) K[AlF3H]
Answer:
(6)K3[AlF6]
Hint: AlF3 + 3KF → K3[AlF6]
Question 16.
Match items in column – I with the items of column – II ans assign the correct code
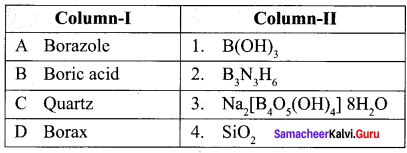
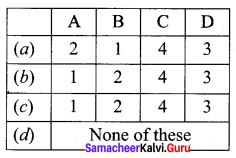
Answer:
(a) A – 2, B – 1, C – 4, D – 3
Question 17.
Duralumin is an alloy of …………
(a) Cu, Mn
(b) Cu, Al, Mg
(c) Al, Mn
(d) Al, Cu, Mn, Mg
Answer:
(d) Al, Cu, Mn, Mg
Hint: Al – 95% , Cu – 4% , Mn – 0.5% , Mg – 1.1 %
Question 18.
Thermodynamically the most stable form of carbon is …………
(a) Diamond
(b) graphite
(c) Fullerene
(d) none of these
Answer:
(b) graphite
Question 19.
The compound that is used in nuclear reactors as protective shields and control rods is …………
(a) Metal borides
(b) metal oxides
(c) Metal carbonates
(d) metal carbide
Answer:
(a) Metal borides
Question 20.
The stability of +1 oxidation state increases in the sequence …………
(a) Al < Ga < In < Tl
(b) Tl < In < Ga < Al
(c) In < Tl < Ga < Al
(d) Ga< In < Al < Tl
Answer:
(a) Al < Ga < In < Tl
II. Answer the following questions:
Question 1.
Write a short note on anamolous properties of the first element of p-block.
Answer:
In p-block elements the first member of each group differs from the other elements of the corresponding group. The following factors are responsible for this anomalous behaviour.
- Small size of the first member.
- High ionisation enthalpy and high electronegativity.
- Absence of d-orbitals in their valance shell.
The first member of the group-13, boron is a metalloid while others are reactive metals. Moreover, boron shows diagonal relationship with silicon of group -14. The oxides of boron and silicon are similar in their acidic nature.
Question 2.
Describe briefly allotropism in p- block elements with specific reference to carbon.
Answer:
Some elements exist in more than one crystalline or molecular forms in the same physical state. This phenomenon is called allotropism. Most common allotropes of carbon are,
- Graphite
- Diamond
- Fullerenes
- Carbon nanotubes
- Graphene.
1. Graphite:
- It is the most stable allotropic form of carbon at normal temperature and pressure.
- It is soft and conducts electricity.
- It is composed of flat two dimensional sheets of carbon atoms.
- Each sheet is a hexagonal net of sp2 hybridised carbon atoms with a C – C bond length of 1.41 A.
- Structure of graphite,
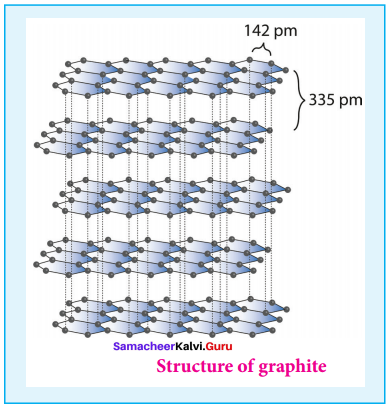
2. Diamond:
- It is very hard.
- The carbon atoms in diamond are sp1 hybridised, with a C – C bond length of 1.54 A.
- In the diamond, carbon atoms are arranged in tetrahedral manner.
- Structure of Diamond,
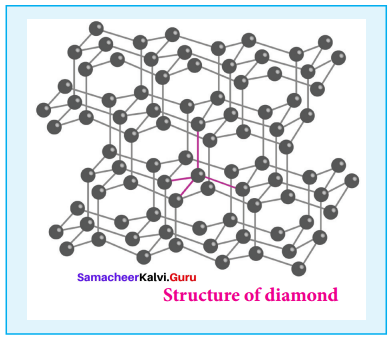
3. Fullerenes:
- It is newly synthesised allotropes of carbon.
- The C60 molecules have a soccer ball like structure and is called buckminster fullerene or buckyballs.
- It has a fused ring structure consists of 20 six membered rings and 12 five membered rings.
- Each carbon atom is sp2 hybridised.
- The C – C bond distance is 1.44 A and C = C distance is 1.38 A.
- Structure of fullerene,
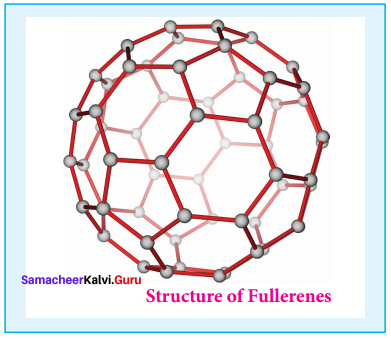
4. Carbon nanotubes:
- It is recently discovered allotropes, have graphite like tubes with fullerene ends.
- These nanotubes are stronger than steel and conduct electricity.
- Structure of Carbon nanotubes.
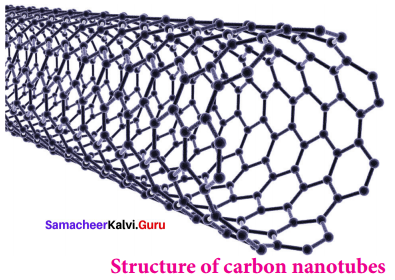
5. Graphene:
- It has a single planar sheet of sp2 hybridised carbon atoms that are densely packed in a honeycomb crystals lattice.
- Structure of Graphene,
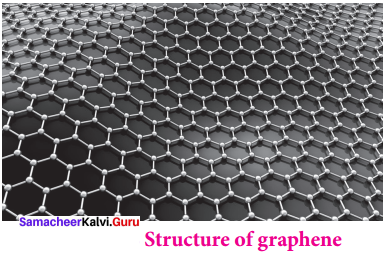
Question 3.
Boron does not react directly with hydrogen. Suggest one method to prepare diborane from BF3.
Answer:
Boron does not react directly with hydrogen. However it forms a variety of hydrides called boranes. Treatment of gaseous boron trifluoride with sodium hydride around 450 K gives diborane.
![]()
Question 4.
Give the uses of Borax.
Answer:
Uses of borax:
- Borax is used for the identification of coloured metal ions.
- In the manufacture optical and borosilicate glass, enamels and glazes for pottery.
- It is also used as a flux in metallurgy and also acts as a good preservative.
Question 5.
What is catenation ? describe briefly the catenation property of carbon.
Answer:
Catenation:
It is the phenomenon of an atom to form a strong covalent bond with the atoms of itself. Carbon shares the property of catenation to maximum extent because it is small in size and can form pn-pn multiple bonds to itself. The following conditions are necessary for catenation.
- The valency of element is greater than or equal to two.
- Element should have an ability to bond with itself.
- The self bond must be as strong as its bond with other elements.
- Kinetic inertness of catenated compound towards other molecules. Carbon possesses all the above properties and forms a wide range of compounds with itself.
Question 6.
Write a note on Fisher tropsch synthesis.
Answer:
The reaction of carbon monoxide with hydrogen at a pressure of less than 50 atm using metal catalysts at 500-700 K yields saturated and unsaturated hydrocarbons.
![]()
Question 7.
Give the structure of CO and CO2.
Answer:
Structure of CO:
![]()
Structure of CO2:
![]()
Question 8.
Give the uses of silicones.
Answer:
Uses of silicones:
- Silicones are used for low temperature lubrication and in vacuum pumps, high temperature oil baths etc.
- They are used for making water proofing clothes.
- They are used as insulting material in electrical motor and other appliances
- They are mixed with paints and enamels to make them resistant towards high temperature, sunlight, dampness and chemicals.
Question 9.
AlCl3 behaves like a lewis acid. Substantiate this statement.
Answer:
In AlCl3, Al in electron deficient it needs two electrons to complete octet so it act as lewis acid. AlCl3 usually exist as a dimer to achieve octet by bridged Cl atom electron deficient compounds are lewis acids.
Question 10.
Describe the structure of diborane.
Answer:
In diborane two BH2 units are linked by two bridged hydrogens. Therefore, it has eight B-H bonds. However, diborane has only 12 valance electrons and are not sufficient to form normal covalent bonds. The four terminal B-H bonds are normal covalent bonds (two centre – two electron bond or 2c-2e bond). The remaining four electrons have to used for the bridged bonds, i.e. two three centred B-H-B bonds utilise two electrons each.
Hence, these bonds are three centre – two electron bonds. The bridging hydrogen atoms are in a plane as shown in the figure. In dibome, the boron is sp3 hybridised. Three of the four sp3 hybridised orbitals contains single electron and the fourth orbital is empty.
Two of the half filled hybridised orbitals of each boron overlap with the two hydrogens to form four terminal 2c-2e bonds, leaving one empty and one half filled hybridised orbitals on each boron. The Three centre – two electron bonds, B-H-B bond formation involves overlapping the half filled hybridised orbital of one boron, the empty hybridised orbital of the other boron and the half filled 1s orbital of hydrogen.
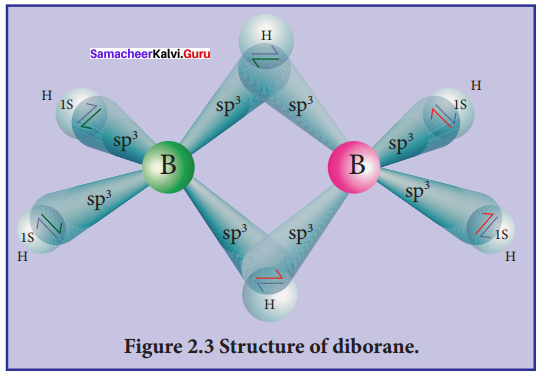
Question 11.
Write a short note on hydroboration.
Answer:
Diborane adds on to alkenes and alkynes in ether solvent at room temperature. This reaction is called as hydroboration and is highly used in synthetic organic chemistry especially for anti-Markovnikov addition.
B2H6 + 3RCH = CHR → B( CH2 – CH2R )3+ 6H2
Question 12.
Give one example for each of the following:
- icosogens
- tetragen
- prictogen
- chalcogen
Answer:
1. Icosogens:
- Boron
- Aluminium
- Gallium
2. Tetragen:
- Carbon
- Silicon
- Germanium
3. Prictogen:
- Oxygen
- Sulfur
- Selenium
4. Chalcogen:
- Fluorine
- Chlorine
- Bromine
Question 13.
Write a note on metallic nature of p-block elements.
Answer:
- The tendency of an element to form a cation by loosing electrons is known as electro¬positive or metallic character.
- This character depends on the ionisation energy.
- Generally on descending a group the ionisation energy decreases and hence the metallic character increases.
In p-block, the elements present in lower left part are metals while the elements in the upper right part are non metals. Elements of group 13 have metallic character except the first element boron which is a metalloid, having properties intermediate between the metal and nonmetals. The atomic radius of boron is very small and it has relatively high nuclear charge and these properties are responsible for its nonmetallic character.
In the subsequent groups the non-metallic character increases. In group 14 elements, carbon is a nonmetal while silicon and germanium are metalloids. In group 15, nitrogen and phosphorus are non metals and arsenic & antimony are metalloids. In group 16, oxygen, sulphur and selenium are non metals and tellurium is a metalloid. All the elements of group 17 and 18 are non metals.
Question 14.
Complete the following reactions:
(a) B(OH)3 + NH3 →
(b) Na2B4O7 + H2SO4+ H2O →
(c) B2H6 + 2NaOH + 2H2O →
(d) B2H6 + CH3OH →
(e) BF3 + 9H2O →
(f) HCOOH + H2SO4→
(g) SiCl4 + NH3 →
(h) SiCl4 + C2H5OH →
(i) B + NaOH →
(j) H2B4O7 \(\underrightarrow { Red\quad hot }\)
Answer:
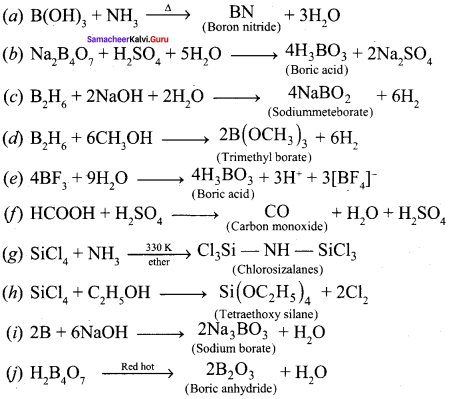
Question 15.
How will you identify borate radical?
Answer:
When boric acid or borate salt is heated with ethyl alcohol in presence of concentrated H2SO4, an ester triethyl borate is formed. The Vapour of this ester burns with a green edged flame and this reaction is used to identify the presence of borate.
![]()
Question 16.
Write a note on zeolites.
Answer:
Zeolites:
- Zeolites are three dimensional crystalline solids containing aluminium, silicon and oxygen in their regular three dimensional framework.
- They are hydrated sodium alumino silicates with general formula, Na2O. (Al2O3). x(SiO2)y(H2O) (x = 2 to 10; y = 2 to 6)
- Zeolites have porous structure in which the monovalent sodium ions and water molecules are loosely held.
- The Si and Al atoms are tetrahederally coordinated with each other through shared oxygen atoms.
- Zeolites structure looks like a honeycomb consisting of a network of interconnected tunnels and cages.
- Zeolite crystal to act as a molecular sieve. They helps to remove permanent hardness of water.
Question 17.
How will you convert boric acid to boron nitride?
Answer:
Fusion of urea with boric acid B(OH)3, in an atmosphere of ammonia at 800 – 1200 K gives
![]()
Question 18.
A hydride of 2nd period alkali metal
(A) on reaction with compound of Boron
(B) to give a reducing agent
(C) identify A, B and C.
Answer:
- A hydride of 2nd period alkali metal (A) is lithium hydride (LiH).
- Lithium hydride (A) reacts with diborane (B) to give lithiumborohydride (C) which is act as reducing agent.
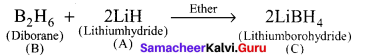
(A) Lithium hydride – LiH
(B) Diborane – B2H6
(C) Lithium borohydride – LiBH4
Question 19.
A double salt which contains fourth period alkali metal
(A) on heating at 500K gives
(B). Aqueous solution of (B) gives white precipitate with BaCl2 and gives a red colour compound with alizarin. Identify A and B.
Answer:
1. A double salt which contains fourth period alkali metal (A) is potash alum
K2SO4. Al2(SO4)3. 24H2O
2. On heating potash alum (A) 500 k give anhydrous potash alum (or) burnt alum (B).

3. Aqueous solution of burnt alum, has sulphates ion, potassium ion and aluminium ion. Sulphate ion reacts with BaCl2 to form white precipitate of Barium Sulphate
(SO4)2 + BaCl2 → BaSO4 + 2Cl–
Aluminium ion reacts with alizarin solution to give a red colour compound.
Question 20.
CO is a reducing agent. Justify with an example.
Answer:
Both thermodynamic and kinetic factors make carbon monoxide (CO) a better reducing agent. When CO is used to reduce a metal oxide, it gets oxidized to CO2 Thermodynamically, CO2 is much more stable than CO. For example,
CO + Fe2O3 → 2Fe + 3CO2
Samacheer Kalvi 12th Chemistry p-Block Elements – I Evaluate yourself
Question 1.
Why group 18 elements are called inert gases? Write the general electronic configuration of group 18 elements.
Answer:
The elements of group-18 have completely filled s and p orbitals, hence they are more stable and have least reactivity. Therefore group-18 elements are called inert gases. ns2np6 is the general electronic configuration of group elements.
Samacheer Kalvi 12th Chemistry p-Block Elements – I Additional Questions
Samacheer Kalvi 12th Chemistry p-Block Elements – I 1 Mark Questions and Answers
I. Choose the correct answer:
Question 1.
More common oxidation state for halogens is …………
(a) +1
(b) +2
(c) -1
(d) -2
Answer:
(c) -1
Question 2.
Electronic configuration of noble gases is …………
(a) ns2
(b) ns2np5
(c) ns1np6
(d) ns2np6
Answer:
(d) ns2np6
Question 3.
Noble gases are chemically inert. This is due to …………
(a) unstable electronic configuration
(b) stable electronic configuration
(c) only filled p-orbital
(d) only filled 5-orbital
Answer:
(b) stable electronic configuration
Question 4.
Consider the following statements.
(i) The first member of the group-13, boron is a metalloid while others are reactive metals.
(ii) The oxides of boron and silicon are similar in their acidic nature.
(iii) Both boron and silicon form metallic hydrides.
Which of the above statement(s) is/are not correct?
(a) (i) only
(b) (ii) only
(c) (ii) and (iii)
(d) (iii) only
Answer:
(d) (iii) only
Question 5.
Which element has a greater tendency to form a chain of bonds with itself?
(a) Boron
(b) Silicon
(c) Tin
(d) Carbon
Answer:
(d) Carbon
Question 6.
Which one of the following is the strongest oxidising agent?
(a) Fluorine
(b) Chlorine
(c) Bromine
(d) Iodine
Answer:
(a) Fluorine
Question 7.
Some elements exist in more than one crystalline or molecular forms in the same physical state is called …………
(a) isomerism
(b) allotropism
(c) isomorphism
(d) isoelectronics
Answer:
(b) allotropism
Question 8.
How many allotropes possible for boron?
(a) 1
(b) 4
(c) 6
(d) 7
Answer:
(c) 6
Question 9.
Important ore of boron is …………
(a) bauxite
(b) borosilicate
(c) borax
(d) P-tetragonal boron
Answer:
(c) borax
Question 10.
Less reactive elements in boron family is …………
(a) Boron
(b) Aluminium
(c) Gallium
(d) Thallium
Answer:
(b) Aluminium
Question 11.
More toxic element in boron family is …………
(a) Boron
(b) Aluminium
(c) Gallium
(d) Thallium
Answer:
(d) Thallium
Question 12.
Boron does not …………
(a) Oxygen
(b) Hydrogen
(c) Acids
(d) Alkali
Answer:
(b) Hydrogen
Question 13.
Borontrifluoride reacts with sodium hydride at 450 K gives …………
(a) diborane
(b) tetraborane
(c) pentaborane
(d) decaborane
Answer:
(a) diborane
Question 14.
2B + N2 \(\underrightarrow { \triangle }\) A . Identify A
(a) BN3
(b) B3N
(c) (BN)3
(d) BN
Answer:
(d) BN
Question 15.
Boron reacts with fused sodium hydroxide to forms …………
(a) Borax
(b) Boric acid
(c) Sodium borate
(d) Sodium tetraborate
Answer:
(c) Sodium borate
Question 16.
Which isotope is used as moderator in nuclear reactors?
(a) 10B5
(b) nC6
(c) 4He2
(d) 40Ca2
Answer:
(a) 10B5
Question 17.
Borax is ………… in nature.
(a) basic
(b) acidic
(c) amphoteric
(d) chemically inert
Answer:
(a) basic
Question 18.
The compound used as a flux in metallurgy?
(a) Boron nitride
(b) Boron oxide
(c) Boron fluoride
(d) Borax
Answer:
(d) Borax
Question 19.
The trialkylborate on reaction with sodiumhydride in tetrahydrofuran to form …………
(a) NaBH4
(b) Na[BH(OR)3]
(c) Na[B(OR)3]
(d) Na[BH(OR)3]
Answer:
(b) Na[BH(OR)3]
Question 20.
Compounds used as an eye lotion …………
(a) H3BO3
(b) HBO2
(c) H2B4O7
(d) B2O3
Answer:
(a) H3BO3
Question 21.
Which one of the following is highly reactive compound?
(a) B2OH3
(b) H2BO3
(c) HBO2
(d) B2H6
Answer:
(d) B2H6
Question 22.
B2H6 + 6CH3OH → A Identify A
(a) B2O3
(b) CH3OB
(c) CH3OH
(d) B(OCH3)3
Answer:
(d) B(OCH3)3
Question 23.
Which one of the following is called as inorganic benzene?
(a) B2H6
(b) BN
(c) H2B4O7
(d) B3N3H6
Answer:
(d) B3N3H6
Question 24.
Compound contains two centred – two electron bond (2c-2e) is …………
(a) B6H11
(b) B5H9
(c) B2H6
(d) B10H11
Answer:
(c) B2H6
Question 25.
Diborane reacts with excess ammonia at high temperature to give …………
(a) Boron nitride
(b) Boron oxide
(c) Borazole
(d) Diborane diammonate
Answer:
(c) Borazole
Question 26.
Consider the following statements.
(i) Diborane contains two centre-two electron bond.
(ii) In diborane, the boron has sp3 hybridis ed.
(iii) Diborane has two terminal B – H bonds and four B – H – B bonds.
Which of the above statement(s) is/are correct.
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) only
(d) (i) and (ii)
Answer:
(d) (i) and (ii)
Question 27.
Compound used for propellant is …………
(a) BN
(b) H2B4O7
(c) B2H2
(d) Borax
Answer:
(c) B2H2
Question 28.
Which one of the following is double salt?
(a) Potash alum
(b) Potassium sulphate
(c) Aluminium Sulphate
(d) Ammonium sulphate
Answer:
(a) Potash alum
Question 29.
Consider the following statements.
(i) Alums are more soluble in hot water than in cold water.
(ii) Alums are more soluble in cold water than in hot water.
(iii) Potash alum is employed a styptic agent to arrest bleeding.
Which of the above statement(s) is/are not correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i), (ii) and (iii)
Answer:
(b) (ii) only
Question 30.
The structure of graphite is …………
(a) planner
(b) hexagonal
(c) octahedral
(d) bucky balls
Answer:
(b) hexagonal
Question 31.
Which one of the following is used as a lubricant?
(a) Graphite
(b) Diamond
(c) Fullerene
(d) Graphene
Answer:
(a) Graphite
Question 32.
Which one of the following carbon allotrope is very hard?
(a) Graphite
(b) Diamond
(c) Fullerene
(d) Graphene
Answer:
(b) Diamond
Question 33.
Recently discovered allotropes of carbon is …………
(a) Graphite
(b) Diamond
(c) Carbon nanotubes
(d) Fullerenes
Answer:
(c) Carbon nanotubes
Question 34.
CO and N2 mixture is …………
(a) natural gas
(b) producer gas
(c) water gas
(d) LPG
Answer:
(b) producer gas
Question 35.
Syn gas is …………
(a) CO + N2
(b) CO + H2
(c) CO2 + H2
(d) CO2 + N2
Answer:
(b) CO + H2
Question 36.
Ethene is mixed with carbon monoxide and hydrogen gas to produce propanal is known as …………
(a) Oxo process
(b) McAfee process
(c) Wacker process
(d) Haber process
Answer:
(a) Oxo process
Question 37.
………… is a good reducing agent.
(a) CO
(b) CO2
(c) [Cr(CO)6]
(d) [Ni(CO)4]
Answer:
(a) CO
Question 38.
Critical temperature of CO2 is ………..
(a) -31°C
(b) -13°C
(c) 31°C
(d) 13°C
Answer:
(c)31°C
Question 39.
Which one of the following compound is important for photosynthesis?
(a) CO
(b) CO2
(c) COCl2
(d) C
Anwer:
(b) CO
Question 40.
General empirical formula of silicone is ………..
(a) (R2SiO)
(b) (RSiO)
(c) (R2CO)
(d) (RSiH)
Answer:
(a) (R2SiO)
Question 41.
Consider the following statements.
(i) All Silicones are hydrophilic in nature.
(ii) Silicones are thermal and electrical insulators.
(iii) Chemically silicones are highly reactive.
Which of the above statement(s) is/are not correct?
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) only
Answer:
(b) (i) and (iii)
Question 42.
Silicate contains ……….. silicon and oxygen in units.
(a) [SiO2]4-
(b) [SiO2]
(c) [SiO2]2-
(d) [SiO2]–
Answer:
(a) [SiO2]4-
Question 43.
Ortho silicates are also called as ………..
(a) Ino silicates
(b) Soro silicates
(c) Neso silicates
(d) Cyclic silicates
Answer:
(c) Neso silicates
Question 44.
Example of Ring silicate is ………..
(a) Olivine
(b) Beryl
(c) Spodumene
(d) Asbestos
Answer:
(b) Beryl
Question 45.
Pick out the three dimensional silicates?
(a) Talc
(b) Mica
(c) Quartz
(d) Asbestos
Answer:
(c) Quartz
Question 46.
Compound used to remove the permanent hardness of water is ………..
(a) Zeolite
(b) Feldspar
(c) Talc
(d) Mica
Answer:
(a) Zeolite
II. Fill in the blanks:
- …………… is the general electronic configuration of tetragen elements.
- Boron and silicon form …………… hydrides.
- …………… is most reactive element among the halogens.
- Most stable oxidation state of aluminium is ……………
- General formula of metal boride is ……………
- Boron has the capacity to absorb ……………
- …………… is used as a rocket fuel igniter.
- …………… is essential for the cell walls of plants.
- …………… is a chemical formula of boron.
- …………… is used for the identification of coloured metal ions.
- …………… is a colourless transparent crystal.
- Boric acid has a …………… structure.
- Boric acid consists of …………… unit.
- On heating magnesium boride with HCl a mixture of volatile …………… are obtained.
- Diborane reacts with methylalcohol to give ……………
- Diborane has …………… B – H bonds.
- In diborane, the boron is …………… hybridised.
- …………… is inorganic benzene.
- Boron trifluoride has a …………… geometry.
- Anhydrous aluminium chloride is a …………… substance.
- With excess of NaOH, aluminium chloride produces ……………
- …………… is used for the manufacture of petrol by cracking the mineral oils.
- …………… is used for water proofing and textiles.
- ……………is the most stable allotropic form of carbon at normal temperature and pressure.
- …………… is allotropic form of carbon has aromatic character.
- Graphene has …………… lattice.
- Carbonyl chloride is a …………… gas.
- Equimolar mixture of hydrogen and carbonmonoxide is called as ……………
- Producer gas is a mixture of …………… and ……………
- Aqueous solution of carbon dioxide forms ……………
- …………… are high temperature polymers.
- The viscosity of silicon oil remains ……………
- …………… are used as insulating material.
- In beryl, each aluminium is surrounded by ……………
- …………… is carcinogenic silicates.
- Three dimensional by replacing units by ……………
- …………… crystal to act as a molecular sieve.
- Borax is a sodium salt of ……………
Answers:
- ns2np2
- covalent
- Fluorine
- +3
- MxBy
- neutrons
- Amorphous boron
- Boron
- Na2B4O7. 10H2O
- Borax
- Boric acid
- two dimensional layered
- [BO3]3-
- borones
- trimethylborate
- eight
- sp3
- Borazine
- planar
- hygroscopic
- Sodium meta aluminate
- AlCF
- Potash alum
- Graphite
- Fullerene
- honey comb nitrogen
- poisonous
- water gas
- carbonmonoxide, nitrogen
- carbonic acid
- silicones
- constant
- Silicones
- six oxygen
- Asbestos
- [SiO4]4-; [AlO4]5-
- Zeolite
- tetraboric acid
III. Match the following:
Question 1.
(i) Tetragens – (a) Oxygen
(ii) Icosagens – (b) Carbon
(iii) Chalcogens – (c) Nitrogen
(iv) Pnictogens – (d) Boron
Answer:
(i) – (b)
(ii) – (d)
(iii) – (a)
(iv) – (c)
Question 2.
(i) Borax – (a) Na2B4O7
(ii) Prismatic form – (b) Na2B4O7. 5H2O
(iii) Jeweller borax – (c) Na2B4O7. 10H2O
(iv) Borax glass – (d) [B4O5(OH)4]2
Answer:
(i) – (c)
(ii) – (d)
(iii) – (b)
(iv) – (a)
Question 3.
(i) Boron – (a) Optical
(ii) Borax – (b) Neutron absorber
(iii) Boric acid – (c) Welding torches
(iv) Diborane – (d) Eye lotion
Answer:
(i) – (b)
(ii) – (a)
(iii) – (d)
(iv) – (c)
Question 4.
(i) Graphene – (a) Honeycomb crystal
(ii) Diamond – (b) Aromatic character
(iii) Fullerene – (c) Lubricant
(iv) Graphite – (d) Very hard
Answer:
(i) – (c)
(ii) – (d)
(iii) – (b)
(iv) – (a)
Question 5.
(i) Ortho silicate
(ii) Pyro silicate
(iii) Cyclic silicate
(iv) Tecto silicate
Answer:
(i) – (d)
(ii) – (a)
(iii) – (b)
(iv) – (c)
IV. Assertion and reason:
Note:
In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) A and R are correct and R explains A.
(b) A and R are correct and R not explains A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Question 1.
Assertion (A) – Noble gases are least reactivity.
Reason (R) – Noble gases have completely filled s and p-orbital and attain stable electronic configuration.
Answer:
(a) A and R are correct and R explains A.
Question 2.
Assertion (A) – Both boron and silicon form covalent hydrides.
Reason (R) – Boron does not shows diagonal relationship with silicon of group 14.
Answer:
(c) A is correct but R is wrong.
Question 3.
Assertion (A) – Fluorine is most reactive element among the halogens.
Reason (R) – Fluorine has minimum bond dissociation energy.
Answer:
(a) A and R are correct and R explains A.
Question 4.
Assertion (A) – Boron combines with halogen to form trihalides at high temperatures.
Reason (R) – Boron does not reacts directly with hydrogen.
(b) A and R are correct and R not explains A.
Question 5.
Assertion (A) – Boron-10 isotope is used as moderator in nuclear reactors.
Reason (R) – Boron has the capacity to absorb neutrons.
Answer:
(a) A and R are correct and R explains A.
Question 6.
Assertion (A) – Diborane is highly reactive.
Reason (R) – At high temperatures, diborane forms higher boranes.
Answer:
(b) A and R are correct and R not explains A.
Question 7.
Assertion (A) – BF3 reacts with ammonia to form complex.
Reason (R) – BF3 is a electron deficient compound and accepts electron pairs to form coordinate covalent bonds.
Answer:
(a) A and R are correct and R explains A.
Question 8.
Assertion (A) – Fullerene has aromatic character.
Reason (R) – Some of the fullerenes have e-bonds and delocalised n-bonds.
Answer:
(a) A and R are correct and R explains A.
Question 9.
Assertion (A) – Carbon dioxide is non flammable gas.
Reason (R) – CO2 critical temperature is 31°C and can be readily liquefied.
Answer:
(b) A and R are correct and R not explains A.
Question 10.
Assertion (A) – Zeolites act as a molecular sieve.
Reason (R) – Zeolite structure is pore/ channel sizes are nearly uniform.
Answer:
(a) A and R are correct and R explains A.
V. Find the odd one out and given the reason:
Question 1.
(a) Icosagens
(b) Tetragens
(c) Alkali metals
(d) Chalcogens
Answer:
(c) Alkali metals
Reason: Alkali metals are s-block elements but other are p-block elements.
Question 2.
(a) Al
(b)B
(c) O
(d) Na
Answer:
(d) Na
Reason: Na is s-block element but others are p-block elements.
Question 3.
(a) Diborane
(b) Borax
(c) Carbonmonoxide
(d) Boric acid
Answer:
(c) Carbonmonoxide
Reason: Carbonmonoxide is not a compound of boron.
Question 4.
(a) B3N3H6
(b) B4H10
(c) B5H9
(d) B5H11
Answer:
(a) B3N3H6
Reason: B3N3H6 not a higher borane
Question 5.
(a) Potash alum
(b) Sodium alum
(c) Burnt alum
(d) Ammonium alum
Answer:
(c) Burnt alum
Reason: Burnt alum does not having water molecule.
Question 6.
(a) Graphite
(b) Borax
(c) Fullerene
(d) Diamond
Answer:
(b) Borax
Reason: Borax is not a allotropic form of carbon.
Question 7.
(a)B
(b) Ga
(c) In
(d) N
Answer:
(d) N
Reason: N is not a icosagens.
VI. Find out the correct pair:
Question 1.
(a) Ortho sililcate – Soro silicate
(b) Pyro silicate – Neso silicate
(c) Chain silicate – Pyroxenes
(d) Double chain silicate – Ring silicate
Answer:
(c) Chain silicate – Pyroxenes
Question 2.
(a) Graphene – 1.54 A
(b) Diamond – 1.40 A
(c) Fullerene – 1.38 A
(d) Graphite – 1.40 A
Answer:
(d) Graphite – 1.40 A
Question 3.
(a) Diborane – welding torches
(b) AlCl3 – Eye lotion
(c) Borax – pigments
(d) Boric acid – optical
Answer:
(a) Diborane – welding torches
Question 4.
(a) Inert gas – ns2np2
(b) Chalcogens – ns2np1
(c) Pnictogens – ns2np4
(d) Tetragens – ns2np4
Answer:
(c) Pnictogens – ns2np3
Question 5.
(a) Icosagen – Oxygen
(b) Tetragen – Carbon
(c) Chalcogen – Fluorine
(d) Halogen – Boron
Answer:
(b) Tetragen – Carbon
VII. Find out the incorrect pair:
Question 1.
(a) Tetragens – ns2np2
(b) Icosagens – ns2np1
(c) Chalcogens – ns2np4
(d) Halogens – ns2np6
Answer:
(d) Halogens – ns2np6
Question 2.
(a) Boron is BF3 – +3
(b) Carbon in CO2 – +4
(c) Nitrogen in N2O5 – +5
(d) Fluorine in OF2 – +4
Answer:
(d) Fluorine in OF2 – +4
Question 3.
(a) Nitrogen – Tetragens
(b) Oxygen – Chalcogens
(c) Tin – Tetragens
(d) Gallium – Icosagens
Answer:
(a) Nitrogen – Tetragens
Question 4.
(a) Boron – Moderator
(b) Borax – Eye lotion
(c) Boric acid – Antiseptic
(d) Diborane – Propellant
Answer:
(b) Borax – Eye lotion
Question 5.
(a) McAfee process – AlCl3
(b) Burnt alum – K2SO4-Al2(SO4)3
(c) Oxo process – Propanal
(d) Fischer tropsch Synthesis – HCOOH
Answer:
(d) Fischer tropsch Synthesis – HCOOH
Question 6.
(a) Soro silicate – Thortveitite
(b) Ring silicate – Beryl
(c) Sheet silicate – Asbestos
(d) Neso silicate – Olivine
Answer:
(c) Sheet silicate – Asbestos
Question 7.
(a) Pyroxenes – Phenacite
(b) Amphiboles – Asbestos
(c) Phyllo silicate – Mica
(d) Tecto silicate – Quartz
Answer:
(a) Pyroxenes – Phenacite
Samacheer Kalvi 12th Chemistry p-Block Elements – I 2 Mark Questions and Answers
Question 1.
What are Wade’s Rule?
Answer:
Wade’s rules are used to rationalize the shape of borane clusters by calculating the total number of skeletal electron pairs (SEP) available for cluster bonding.
Question 2.
Why-1 oxidation state is more common in halogens. Explain.
Answer:
The halogens have a strong tendency to gain an electron to give a stable halide ion with completely filled electronic configuration (ns2sp6) and hence-1 oxidation state is more common in halogens.
Question 3.
Why boron has non-metallic character?
Answer:
The atomic radius of boron is very small and it has relatively high nuclear charge and these properties are responsible for its non-metallic character.
Question 4.
Define inert pair effect.
Answer:
In heavier post-transition metals, the outer s-electron(m) have a tendency to remain inert and show reluctance to take part in the bonding (only p-orbital involved in chemical bonding), which is known as inert pair effect.
Question 5.
Mention the allotropes of boron.
Answer:
- Amorphous boron
- a-rhombohedral boron
- p-rhombohedral boron
- γ – rhombohedral boron
- α – tetragonal boron
- β – tetragonal boron
Question 6.
List out the allotropes of tin.
Answer:
- Grey tin
- White tin
- Rhombic tin
- Sigma tin
Question 7.
Mention the allotropes of phosphorous?
Answer:
- White phosphorous
- Red phosphorous (v)
- Scarlet phosphorous
- Violet phosphorous
- Black phosphorous
Question 8.
Give the two allotropes of sulphur.
Answer:
- Rhombus
- Monoclinic
Question 9.
Why boron compounds are covalent in nature?
Answer:
Many of boron compounds are electron deficient and has unusual type of covalent bonding, which is due to its small size, high ionisation energy and similarity in electronegativity with carbon and hydrogen.
Question 10.
Define Borax is basic in nature.
Answer:
Borax is basic in nature and its solution in hot-water is alkaline as it dissociates into boric acid and sodium hydroxide,
Na2B4O7 + 7H2O → 4H3BO3 + 2NaOH
Question 11.
Explain action of heat on borax.
Answer:
On heating borax, it forms a transparent borax beads.
![]()
Question 12.
What happen when borax treated with ammonium chloride?
Answer:
When borax treated with ammonium chloride, it forms boron nitride.
![]()
Question 13.
What happen when boric acids reacts with sodium hydroxide?
Answer:
Boric acid reacts with sodium hydroxide to form sodium metaborate and sodium tetraborate.
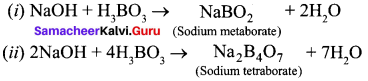
Question 14.
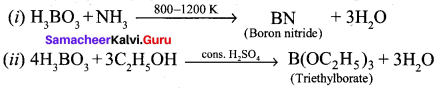
Identify A and B from the following reaction,
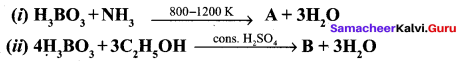
Answer:
Question 15.
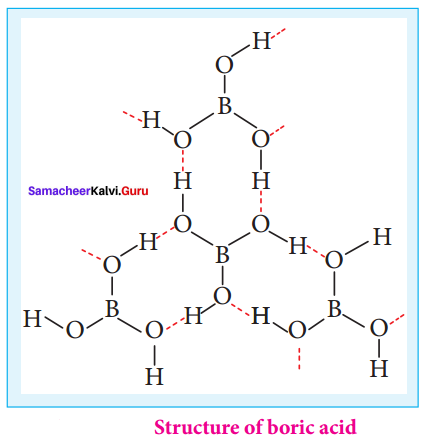
Explain the action of air on diborane.
Answer:
At room temperature pure diborane does not react with air or oxygen but in impure form it gives [B2O3]3- along with large amount of heat.
Question 16
Explain the action of air on diborane.
Answer:
At room temperature pure diborane does not react with air or oxygen but in impure form it gives B2O3 along with large amount of heat.
B2H6+ 3O2 → B2O3 + 3H2O
Question 17.
How will you convert diborane into sodium borohydride?
Answer:
Diborane reacts with sodium hydride in the presence of diglyme to give sodim borohydride.

Question 18.
Mention the uses o boron trifluoride.
Answer:
- Boron trifluoride is used for preparing HBF4, a catalyst in organic chemistry
- It is also used as a fluorinating reagent.
Question 19.
What is MCA fee process?
Answer:
Aluminium chloride is obtained by heating a mixture of alumina and coke in a current of chloride.
![]()
Question 20.
What happen when potash alum is treated with ammonium hydroxide?
Answer:
Potash alum forms aluminium hydroxide, when, treated with ammonium hydroxide
K2SO4. A12(SO4)3. 24H2O + 6NH4OH → K2SO4 + 3(NH4)2SO4 + 24H2O + 3Al(OH)3
Question 21.
What is producer gas? How will you prepare producer gas?
Answer:
On industrial scale carbon monoxide is produced by the reaction of carbon with air. The carbon monoxide formed will contain nitrogen gas also and the mixture of nitrogen and carbon monoxide is called producer gas.
![]()
Question 22.
What is synthetic gas?
Answer:
A mixture of carbonmonoxide and hydrogen is called synthetic gas.
Question 23.
What is phosgene?
Answer:
When carbon monoxide is treated with chlorine in presence of light or charcoal, it forms a poisonous gas carbonyl chloride, when is also known as phosgene.
![]()
Question 24.
How will you prepare propanal by oxoprocess?
Answer:
In oxoprocess, ethene is mixed with carbon monoxide and hydrogen gas to produce propanal.
![]()
Question 25.
What are water gas equilibrium?
Answer:
The equilibrium involved in the reaction between carbon dioxide and hydrogen, has many industrial applications and is called water gas equilibrium.
![]()
Question 26.
Mention the uses of silicon tetrachloride.
Answer:
- Silicon tetrachloride is used in the production of semiconducting silicon.
- It is used as a starting material in the synthesis of silica gel, silicic esters, a binder for ceramic materials.
Question 27.
What are silicones?
Answer:
Silicones or poly siloxanes are organo silicon polymers with general empirical formula (R2SiO). Since their empirical formula is similar to that of ketone (R2CO), they were named silicones.
Question 28.
What are high-temperature polymers?
Answer:
Silicones are high-temperature polymers, because they have high thermal stability.
Question 29.
Why silicones are water repellent?
Answer:
All silicones are water repellent, this is due to the presence of organic side groups that surrounds the silicon which makes the molecule looks like an alkane. Therefore silicones are water repellent.
Samacheer Kalvi 12th Chemistry p-Block Elements – I 3 Marks Questions and Answers
Question 1.
Write a notes on ionisation enthalpy in p-block elements?
Answer:
1. As we move down a group, generally there is a steady decrease in ionisation enthalpy of elements due to increase in their atomic radius.
2. In p-block elements there are some minor deviations to this general trend. In group 13, from B to Al the ionisation enthalpy decreases as expected. But from Al to Tl there is only a marginal difference. This is due to the presence of inner d- and f-elements which has poor shielding effect compared to s and p electrons. As a result, the effective nuclear charge on the valance electrons increase.
3. A similar trend is also observed in group 14. The remaining groups (15-18) follows the general trend, in these groups the ionisation enthalpy decreases as we move down the group. Here poor shielding effect of d- and f-electrons are overcome by the increased shielding effect of the additional p-electrons.
4. The ionisation enthalpy of elements in successive groups is higher than the corresponding elements of the previous group as expected.
Question 2.
Give the uses of boron.
Answer:
- Boron has the capacity to absorb neutrons. Hence, its isotope 10B5 is used as moderator in nuclear reactors.
- Amorphous boron is used as a rocket fuel igniter.
- Boron is essential for the cell walls of plants.
- Compounds of boron have many applications. For example eye drops, antiseptics, washing powders etc..contains boric acid and borax. In the manufacture of Pyrex glass , boric oxide is used.
Question 3.
How will be prepare borax from colemanite?
Answer:
Borax is a sodium salt of tetraboric acid. It is obtained from colemanite ore by boiling its solution with sodium carbonate.
![]()
Question 4.
Explain the extraction of boric acid from,
-
- Borax
- Colemanite
Answer:
1. Borax – Borax is treated with hydrochloric acid or sulphuric acid or nitric acid to give boric acid.
![]()
2. Colemanite – Colemanite is boiling with water, to give boric acid.
![]()
Question 5.
Explain the action of heat on boric acid.
Answer:
Boric acid when heated at 373 K gives metaboric acid and at 413 K, it gives tetraboric acid. When heated at red hot, it gives boric anhydride which is a glassy mass.
- 4 H3BO3 \(\underrightarrow { 373k }\) 4HBOz + H2O
- 4HBO2 \(\underrightarrow { 413k }\) H2B4O7 + H2O
- H2B4O7 \(\underrightarrow { Red hot }\) 2B2O3 + H2O
Question 6.
How will you convert, boric acid into,
- Boron trifluoride
- Borax
Answer:
1. Boron trifluoride:
Boric acid reacts with calcium fluoride in presence of cone. Sulphuric acid and gives boron trifluoride.
![]()
2. Borax:
Boric acid, when heated with soda ash it gives borax.
![]()
Question 7.
Mention the use of boric acid.
Answer:
- Boric acid is used in the manufacture of pottery glazes, glass, enamels and pigments.
- It is used as an antiseptic and as an eye lotion.
- It is also used as a food preservative.
Question 8.
Explain the preparation of diborane.
Answer:
1. Diborane can also be obtained in small quantities by the reaction of iodine with sodium borohydride in diglyme.
2NaBH4 + I2 → B2H6 + 2NaI + H2
2. On heating magnesium boride with HCl a mixture of volatile boranes are obtained.
- 2Mg3B2+ 12HCl → 6MgCl2 + B4H10 + H2
- B4H10+ H2 → 2B2Hg (Diborane)
Question 9.
What happen when diborane heated at various temperature?
Answer:
Diboranc is a gas at room temperature with sweet smell and it is extremely toxic. It is also highly reactive. At high temperatures it forms higher boranes liberating hydrogen.
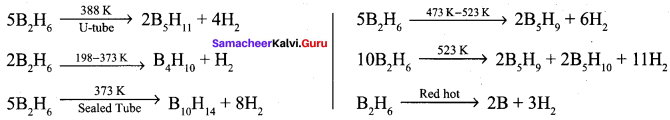
Question 10.
Explain the reaction between diborane and ammonia?
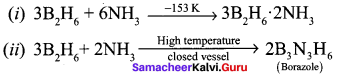
Answer:
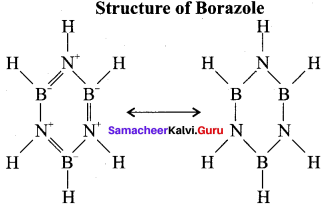
When treated with excess ammonia at low temperatures diborane gives diborane – diammonate on heating at higher temperature diborane gives borazole.
Question 11.
Why borontrifluoride is act as lewis acid? Explain the react between BF3 and ammonia?
Answer:
BF3 is an electron deficient compound and accepts electron pairs to form coordinate covalent bonds. Therefore, borontrifluoride is act as lewis acid. When BF3 reacts with ammonia to form complex.
![]()
Question 12.
How does AlCl3 reacts with following reagents?
- H2O
- NH4OH
- NaOH
Answer:
1. When aluminium chloride reacts with water to form aluminium hydroxide.
AlCl3 + 3H2O → Al(OH)3 + 3HCl
2. With ammonium hydroxide, aluminium chloride forms aluminium hydroxide.
AlCl3 + 3NH7OH → Al(OH)3 + 3NH4C1
3. With excess of sodium hydroxide AlCl3 produces sodium meta aluminate.
AlCl3 + 4NaOH → NaAlO2 + 2H2O + 3NaCl
Question 13.
Give the uses of aluminium chloride.
Answer:
- Anhydrous aluminium chloride is used as a catalyst in Friedels Crafts reactions.
- It is used for the manufacture of petrol by cracking the mineral oils.
- It is used as a catalyst in the manufacture on dyes, drugs and perfumes.
Question 14.
How will you prepare potash alum?
Answer:
The alunite the alum stone is the naturally occurring form and it is K2SO4. A12(SO4)3. 4Al(OH)3. When alum stone is treated with excess of sulphuric acid, the aluminium hydroxide is converted to aluminium sulphate. A calculated quality of potassium sulphate is added and the solution is crystallised to generate potash alum. It is purified by recrystallisation.
K2SO4. A12(SO4)3. 4Al(OH)3 + 6H2SO4 → K2SO4 + Al2(SO 4)3 + 12H2O
K2SO4 + A12(SO4)2 + 24H2O → K2SO4. A12(SO4)3. 24H2O
Question 15.
Mention the uses of the potash alum.
Answer:
- It is used for purification of water
- It is also used for water proofing and textiles
- It is used in dyeing, paper and leather tanning industries
- It is employed as a styptic agent to arrest bleeding.
Question 16.
What is burnt alum?
Answer:
When potash alum is heated at 365 K. It melts and form molten state. Again heated at 475 K they lose water of hydration and swells up. Thee swollen mass is known as burnt alum.
Question 17.
What are all the conditions are necessary for catenation?
Answer:
Essential condition for catenation:
- The valency of elements is greater than or equal to two.
- Element should have an ability to bond with itself.
- The self bond must be as strong as its bond with other elements.
- Kinetic inertness of catenated compound towards other molecules.
Question 18.
Give any three methods to prepare carbon monoxide.
Answer:
1. Carbon monoxide can be prepared by the reaction of carbon with limited amount of oxygen.
2C + O2 → 2CO
2. On industrial scale carbon monoxide is produced by the reaction of carbon with air.
![]()
3. Pure carbon monoxide is prepared by warming methanoic acid with concentrated sulphuric acid which act as a dehydrating agent.
HCOOH + H2SO4 → CO + H2O + H2SO4
Question 19.
Mention the uses the carbon monoxide.
Answer:
- Equimolar mixture of hydrogen and carbon monoxide – water gas and the mixture of carbon monoxide and nitrogen – producer gas are important industrial fuels
- Carbon monoxide is a good reducing agent and can reduce many metal oxides to metals.
- Carbon monoxide is an important ligand and forms carbonyl compound with transition metals
Question 20.
Explain the methods to prepare carbon dioxide.
Answer:
1. On industrial scale it is produced by burning coke in excess of air.
![]()
2. Calcination of lime produces carbon dioxide as by product.
CaCO3 \(\underrightarrow { \triangle }\) CaO + CO2
3. Carbon dioxide is prepared in laboratory by the action of dilute hydrochloric acid on metal carbonates.
CaCO2 + 2HCl → CaCl2 + H2O + CO2
Question 21.
Give the uses of carbon dioxide.
Answer:
- Carbon dioxide is used to produce an inert atomosphere for chemical processing.
- Biologically, it is important for photosynthesis.
- It is also used as fire extinguisher and as a propellent gas.
- It is used in the production of carbonated beverages and in the production of foam.
Question 22.
Explain the preparation of silicon tetrachloride.
Answer:
1. Silicon tetrachloride can be prepared by passing dry chlorine over an intimate mixture of silica and carbon by heating to 1675 K in a porcelain tube.
SiO2 + 2C + 2Cl2 → SiCl2 + 2CO
2. On commercial scale, reaction of silicon with hydrogen chloride gas occurs above 600 K.
Si + 4HCl → SiCl4 + 2H2
Question 23.
Complete the following reaction,
- SiCl4 + H2O → ?
- SiCl4 + C2HSOH → ?
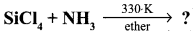
Answer:
- SiCl4 + 4H2O → Si(OH)4 + 4HCl
- SiCl4 + C2H5OH → Si(OC2H5)4+ 2C12
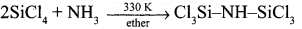
Question 24.
Explain the types of silicones.
Answer:
- Linear silicones – They are obtained by the hydrolysis and subsequent condensation of dialkyl or diaryl silicon chlorides.
- Silicone rubbers – These silicones are bridged together by methylene or similar groups.
- Silicone resins – They are obtained by blending silicones with organic resins such as acrylic esters.
- Cyclic silicones – These are obtained by the hydrolysis of R2SiCl2.
- Cross linked silicones – They are obtained by hydrolysis of RSiClr
Question 25.
Mention the properties of silicones.
Answer:
- All silicones are water repellent.
- They are thermal and electrical insulators.
- Chemically they are inert.
- Lower silicones are oily liquids whereas higher silicones with long chain structure are waxy solids.
- The viscosity of silicon oil remains constant and doesn’t change with temperature and they don’t thicken during winter.
Question 26.
What are silicates and mention the types of silicates?
Answer:
Silicates:
The mineral which contains silicon and oxygen in tetrahedral [\({ { [S{ i }{ O }_{ 4 }] } }^{ 4- }\) units linked together in different patterns are called silicates.
types of Silicates:
- Ortho silicates
- Pyro silicates
- Cyclic silicates
- Ino silicates
- Phyllo silicates
- Tecto silicates
Samacheer Kalvi 12th Chemistry p-Block Elements – I 5 Mark Questions and Answers
Question 1.
An element (A) extracted from kernite. A reacts with nitrogen at high temperature gives B. A reacts with alkali to form C. Find out A, B and C. Give the chemical equations.
Answer:
1. An element (A) extracted form kemite is boron.
2. Boron reacts with nitrogen at high temperature gives Boron nitride (B)
![]()
3. Boron reacts with alkali (NaOH) to gives sodium borate (C).
![]()

Question 2.
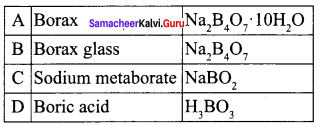
Compound A is used in the manufacture of opticals. A on heating gives B. B further heating to form C. A reacts with hydrochloric acid to give D. Identify A, B and C. Explain the reaction.
Answer:
1. Compound (A) is borax, which is used in the manufacture of opticals.
2. Borax (A) on heating to give borax glass (B). Borax glass on further heating to give sodium metaborate (C).
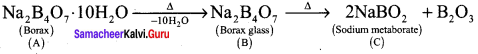
3. Borax (A) reacts with hydrochloride acid to give boric acid.
![]()
Question 3.
Complete the reaction.
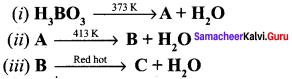
Answer:
Boric acid when heated at 373 K gives metaboric acid (A) and A heated at 413 K it gives tetraboric acid (B). B heated at red hot it gives boric anhydride (C).
![]()

Question 4.
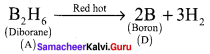
Sodium borohydride reacts with iodine in the presence of diglyme to give A. A heated at 388 K give B. A heated at 373 K in sealed tube to form C. A further heated at red hot condition to give element D. Find out A, B, C and D. Give the reactions.
Answer:
1. Sodium borohydride reacts with iodine in the presence of diglyme to give diborane (A).
![]()
2. Diborane (A) heated at 388 K gives pentaborane (11) (B).

3. Diborane (A) heated at 373 K in the sealed tube to form decaborane
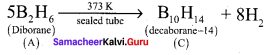
(14) (C).Diborane (A) heated at red hot conditions to give element boron (D) Diborane B2H6

Question 5.
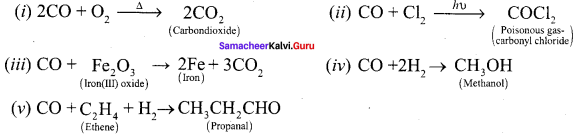
How does carbonmonoxide reacts with following reagents,
(i) O2
(ii) Cl2
(iii) F2O3
(iv) H2
(v) ethene
Answer:
Question 6.
Explain the preparation of silicones.
Answer:
Generally silicones are prepared by the hydrolysis of dialkyldichiorosilanes (R2SiCl2) or diaryldichlorosilanes Ar2SiCl2, which are prepared by passing vapours of RCl or ArCl over siliconat 570 K with copper as a catalyst.
2RCl + Si \(\underrightarrow { Cu/570k }\) R2SiCl2
The hydrolysis of dialkvlchloro silanes R2SiCl2 yields to a straight chain polymer which grown from both the sides
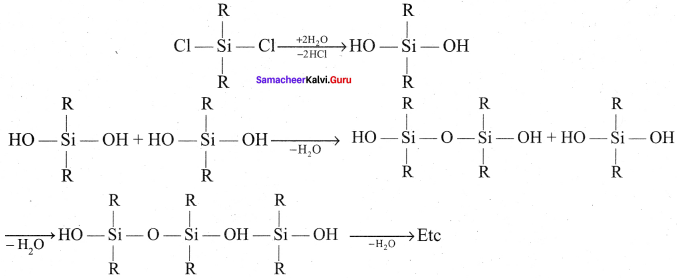
The hydrolysis of monoalkylchloro silanes RSiCl3, yields to a very complex cross linked polymer Linear silicones can be converted into cyclic or ring silicones when water molecules is removed
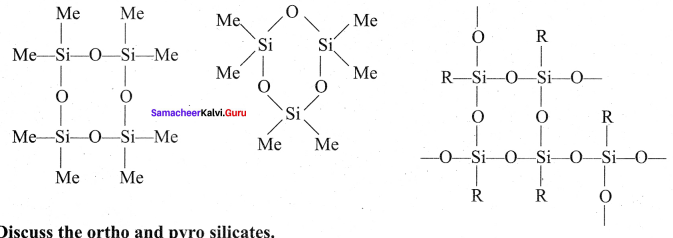
Question 7.
Discuss the ortho and pyro silicates.
Answer:
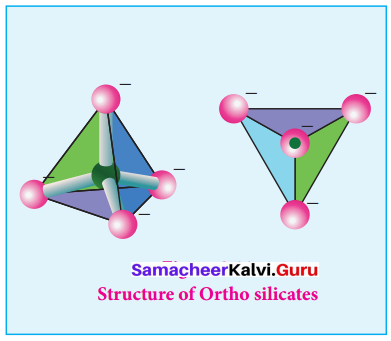
Ortho silicates:
The simplest silicates which contain discrete [SiO4]4- tetrahedral units are called ortho silicates or neso silicates.
Examples:
Phenacite – Be2SiO4(Be2+) ions are tetrahedrally surrounded by O2- ions). Olivine – (Fe/Mg)2SiO4( Fe2+ and Mg2+ cations are octahedrally surrounded by O2-ions).
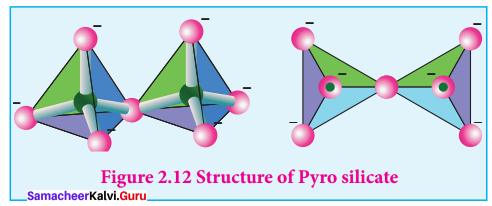
Pyro silicates:
Silicates which contain [Si2O7]6- ions are called pyro silicates (or) Soro silicates They are formed by joining two [SiO4]4- tetrahedral units by sharing one oxygen atom at one comer, (one oxygen is removed while, joining).
Example:
Thortveitite – SC2Si2O7.
Question 8.
Explain:
- Cyclic silicates
- Ino silicates
Answer:
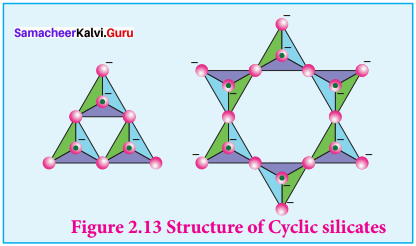
1. Cyclic silicates:
Silicates which contain \(({ Si{ O }_{ 3 }) }_{ n }^{ 2n- }\) ions which are formed by linking three or more tetrahedral \(({ Si{ O }_{ 3 }) }_{ n }^{ 4- }\) units cyclically are called cyclic silicates. Each silicate unit shares two of its oxygen atoms with other units.
Example:
Beryl [Be3Al2(SiO3)6] (an aluminosilicate with each aluminium is surrounded by 6 Structure of Cyclic silicates
2. Ino silicones:
Silicates which contain V number of silicate units liked by sharing two or more oxygen atoms are called inosilicates. They are further classified as chain silicates and double chain silicates. Chain silicates (or pyroxenes) – These silicates contain \(({ Si{ O }_{ 3 }) }_{ n }^{ 2n- }\) ions formed by linking ‘n’ number of tetrahedral \(({ Si{ O }_{ 3 }) }_{ n }^{ 4- }\) units linearly. Each silicate unit shares two of its oxygen atoms with other Structure of Chain silicates units.
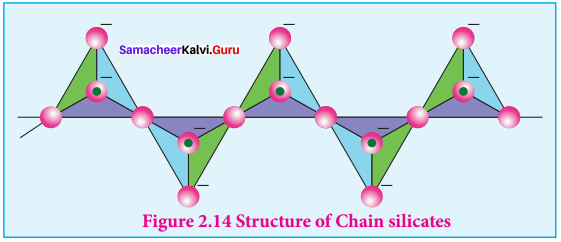
Example:
Spodumene – LiAl(SiO3)2 Double chain silicates (or amphiboles) – These silicates contains \([{ S{ i }_{ 4 }{ O }_{ 11 }] }_{ n }^{ 6n- }\) ions. In these silicates there are two different types of
3. Tetrahedra:
- Those sharing 3 vertices
- Those sharing only 2 vertices.
Examples.
Asbestos – These are fibrous and noncombustible silicates. Therefore they are used for thermal insulation material, brake linings, construction material and filters. Asbestos being carcinogenic silicates, their applications are restricted.
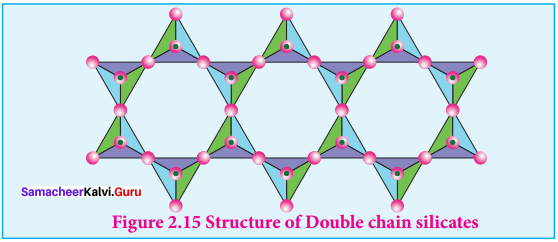
Question 9.
Write a notes on
- Sheet silicates
- Three dimensional silicate
Answer:
1. Sheet silicates:
Silicates which contain \(({ S{ i }_{ 2 }{ O }_{ 5 }) }_{ n }^{ 2n- }\) are called sheet or phyllo silicates. In these, Each \({ { [S{ i }{ O }_{ 4 }] } }^{ 4- }\) tetrahedron unit shares three oxygen atoms with others and thus by forming twodimensional sheets. These sheets, silicates form layered structures in which silicate sheets are stacked over each other. The attractive forces between these layers are very week, hence they can be cleaved easily just like graphite.
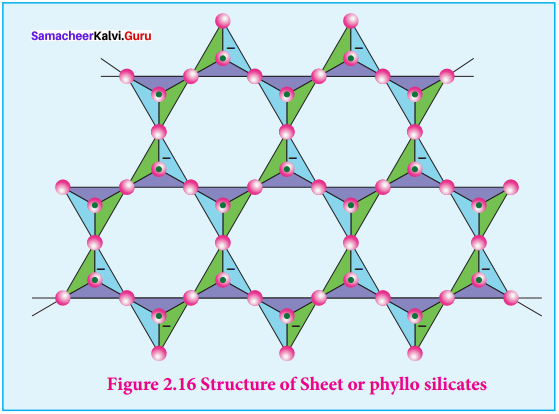
Example: Talc, Mica etc…
2. Three dimensional silicate:
Silicates in which all the oxygen atoms of \({ { [S{ i }{ O }_{ 4 }] } }^{ 4- }\) tetrahedra are shared with other tetrahedra to form three-dimensional network are called three dimensional or tecto silicates. They have general formula (SiO2)n.
Examples:
Quartz. These tecto silicates can be converted into Three dimentional aluminosilicates by replacing [\({ { [S{ i }{ O }_{ 4 }] } }^{ 4- }\) – units by [AlO4]5- units. E.g. Feldspar, Zeolites etc.,
Question 10.
Explain – Boron neutron capture therapy.
Answer:
1. The affinity of boron-10 for neutrons is the basis of a technique known as born neutron capture therapy (BNCT) for treating patients suffering from brain tumours.
2. It is based on the nuclear reaction that occurs when boron-10 is irradiated with low- energy thermal neutoms to give high linear energy a-particles and a Li-particles.
3. Boron compounds are injected into a patient with a brain tumour and the compounds collect preferentially in the tumour. The tumour area is then irradiated with thermal neutom and results in the release of an alpha-particle that damages the tissue in the tumour each time a boron-10 nucleus captures a neutron.
3. In this way damages can be limited preferentially to the tumour, leaving the normal brain tissue less affected.
4. BNCT has also been studied as a treatment for several other tumours of the head and neck, The breast, the prostate, the bladder and the liver.
Commn Errors and its Rectifications
Common Errors:
- Ores formula may be difficult to remember.
- Allotropes of elements may confuse the students in writing equation.
Rectifications:
- Only chief ore from which the element is extracted can be easy to remember.
- Crystalline forms are more chemically reactive.
We hope the Class 12th Samacheer Kalvi Chemistry Solutions Chapter 2 p-Block Elements – I Book Solutions Answers Guide Solutions provided here help the students to get the best knowledge. Also, students can use our Samacheer Kalvi Class 12th Chemistry Solutions Chapter 2 p-Block Elements – I Questions and Answers for better practice. You can contact us at any moment by giving comments in the comment section. Keep checking updates by bookmarking our website.