Tamilnadu State Board Solutions for Class 12th Chemistry Solutions Chapter 5 Coordination Chemistry Questions and Answers will help you to improve the complete subject knowledge. Every student must look at the single concept included in Samacheer Kalvi 12th Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions Answers Guide Solutions PDF. All the concepts are explained clearly with examples and pictures.
Students can easily avoid the struggle to get the best book for Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions Answers Guide learning. You have to go through Chapterwise Samacheer Kalvi Class 12th Textbook Solutions for Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions for better practice.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 5 Coordination Chemistry
Students who wish to have the strong basics of Chemistry Solutions Chapter 5 Coordination Chemistry can use Tamilnadu State Board Class 12th Chemistry Solutions Chapter 5 Coordination Chemistry Questions and Answers Guide pdf. Enhance your knowledge by referring to the Samacheer Kalvi Solutions pdf. We provided a free pdf of Tamilnadu State Board Class 12th Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions Answers Guide material and textbook for students. Check it out now and start preparing for the exam immediately. Score maximum marks in the exam by referring to Samacheer Kalvi Class 12th Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions Answers Guide Solutions Pdf.
Samacheer Kalvi 12th Chemistry Coordination Chemistry TextBook Evalution
I. Choose the correct answer.
12th Chemistry Chapter 5 Book Back Answers Questions 1.
The sum of primary valance and secondary valance of the metal M in the complex [M(en)2(Ox)]Cl is ……………..
(a) 3
(b) 6
(c) -3
(d) 9
Answer:
(d) 9
12th Chemistry Chapter 5 Evaluate Yourself Answers Question 2.
An excess of silver nitrate is added to 100ml of a 0.01M solution of penta aquachlorido chromium (III) chloride. The number of moles of AgCl precipitated would be ……………..
(a) 0.02
(b) 0.002
(c) 0.01
(d) 0.2
Answer:
(b) 0.002
12th Chemistry 5th Lesson Evaluate Yourself Answers Question 3.
A complex has a molecular formula MSO4Cl. 6H2O. The aqueous solution of it gives white precipitate with Barium chloride solution and no precipitate is obtained when it is treated with silver nitrate solution. If the secondary valence of the metal is six, which one of the following correctly represents the complex?
(a) [M(H2O)4Cl] SO2. 2H22O
(b) [M(H2O)6] SO4
(C)[M(H2O)5Cl] SO4. H2O
(d) [M(H2O)3Cl] SO4. 3H2O
Answer:
(c) [M(H2O)5Cl]SO4. H2O
Coordination Chemistry Questions And Answers Pdf Question 4.
Oxidation state of Iron and the charge on the ligand NO in [Fe(H2O)5NO] SO4 are ……………..
(a) +2 and 0 respectively
(b) +3 and 0 respectively
(c) +3 and -1 respectively
(d) +1 and +1 respectively
Answer:
(d) +1 and +1 respectively
12th Chemistry 5th Lesson Question 5.
As per IUPAC guidelines, the name of the complex [CO(en)2(ONO)Cl]Cl is ……………..
(a) chlorobisethylenediaminenitritocobalt (III) chloride
(b chloridobis (ethane-1, 2-diamine) nitro k – Ocobaltate (III) chloride
(c) chloridobis (ethane-1, 2-diammine) nitrito k – Ocobalt (II) chloride
(d) chloridobis (ethane-1, 2-diamine) nitro k – Ocobalt (III) chloride
Answer:
(d) chloridobis (ethane-1, 2-diamine) nitro k – Ocobalt (III) chloride
12 Chemistry Evaluate Yourself Answers Question 6.
IUPAC name of the complex K3[Al(C2O4)3] is ……………..
(a) potassiumtrioxalatoaluminium (III)
(b) potassiumtrioxalatoaluminate (II)
(c) potassiumtrisoxalatoaluminate (III)
(d) potassiumtrioxalatoaluminate (III)
Answer:
(d) potassiumtrioxalatoaluminate (III)
12th Chemistry Evaluate Yourself Answers 2021 Question 7.
A magnetic moment of 1.73BM will be shown by one among the following ……………..
(a) TiCl4
(b) [COCl6]4-
(c) [Cu(NH3)4]2+
(d) [Ni(CN)4]2-
Answer:
(c) [Cu(NH3)4]2+
12th Chemistry Evaluate Yourself Answers Question 8.
Crystal field stabilization energy for high spin d5 octahedral complex is ……………..
(a) – 0.6∆0
(b) 0
(c) 2 (P – ∆0)
(d) 2 (P + ∆0)
Answer:
(b) 0
12th Chemistry Lesson 5 Book Back Answers Question 9.
In which of the following coordination entities the magnitude of ∆0 will be maximum?
(a) [CO(CN)6]3-
(b) [CO(C2O4)3]3-
(c) [CO(H2O)6]3+
(d) [CO(NH3)6]3+
Answer:
(a) [CO(CN)6]3-
Coordination Compounds Important Questions With Solutions Pdf Question 10.
Which one of the following will give a pair of enantiomorphs?
(a) [Cr(NH3)6][CO(CN)6]
(b) [CO(en)2Cl2]Cl
(c) [Pt(NH3)4][PtCl4]
(d) [CO(NH3)4Cl2]NO2
Answer:
(b) [CO(en)2Cl2]Cl
Important Questions Of Coordination Compounds Class 12 Question 11.
Which type of isomerism is exhibited by [Pt(NH3)2Cl2] ?
(a) Coordination isomerism
(b) Linkage isomerism
(c) Optical isomerism
(d) Geometrical isomerism
Answer:
(d) Geometrical isomerism
Samacheer Kalvi Guru 12th Chemistry Question 12.
How many geometrical isomers are possible for [ Pt (Py) (NH3) (Br) (Cl) ]?
(a) 3
(6) 4
(c) 0
(d) 15
Answer:
(a) 3
12th Chemistry Samacheer Kalvi Question 13.
Which one of the following pairs represents linkage isomers?
(a) [Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
(b) [CO(NH3)5(NO3)]SO4 and [CO(NH3)5(ONO)]
(c) [CO(NH3)4(NCS)2]Cl and [CO(NH3)4(SCN)2]Cl
(d) both (b) and (c)
Answer:
(c) [CO(NH3)4(NCS)2]Cl and [CO(NH3)4(SCN)2]Cl
12th Chemistry Evaluate Yourself Answers Samacheer Question 14.
Which kind of isomerism is possible for a complex [CO(NH3)4Br2]Cl ?
(a) geometrical and ionization
(b) geometrical and optical
(c) optical and ionization
(d) geometrical only
Answer:
(a) geometrical and ionization
Chemistry Samacheer Kalvi Question 15.
Which one of the following complexes is not expected to exhibit isomerism?
(a) [Ni(NH3)4(H2O)2]2+
(b) [Pt(NH3)2 Cl2]
(C) [CO(NH3)5SO4]Cl
(d) [Fe(en)3]3+
Answer:
(d) [Fe(en)3]3+
Samacheerkalvi.Guru 12th Chemistry Question 16.
A complex in which the oxidation number of the metal is zero is ……………..
(a) K4[Fe(CN)6]
(b) [Fe(CN)3(NH3)3]
(c) [Fe(CO)5]
(d) both (b) and (c)
Answer:
(c) [Fe(CO)5]
Samacheer Kalvi 12th Chemistry Question 17.
Formula of tris (ethane-1, 2-diamine) iron (II) phosphate ……………..
(a) [Fe(CH3 – CH(NH2)2)3] (PO4)3
(b) [Fe(H2N – CH2 – CH2 – NH2)3] (PO4)
(c) [Fe(H2N – CH2 – CH2 – NH2)3](PO4)2
(d) [Fe(H2N – CH2 – CH2 – NH2)3](PO4)2
Answer:
(d) [Fe(H2N – CH2 – CH2 – NH2)3](PO4)2
12 Chemistry Samacheer Kalvi Question 18.
Which of the following is paramagnetic in nature?
(a) [Zn(NH3)4]2+
(b) [CO(NH3)6]3+
(c) [Ni(H2O)6]2+
(d) [Ni(CN)4]2-
Answer:
(c) [Ni(H2O)6]2+
Samacheer Kalvi Chemistry Question 19.
Facmer isomerism is shown by ……………..
(a) [CO(en)3]3+
(b) [CO(NH3)4(Cl)2]+
(c) [CO(NH3)3(Cl)3]
(d) [CO(NH3)5Cl]SO4
Answer:
(c) [CO(NH3)3(Cl)3]
Coordination Compounds Class 12 Notes Question 20.
Choose the correct statement.
(a) Square planar complexes are more stable than octahedral complexes
(b) The spin only magnetic moment of [Cu(Cl)4]2- is 1.732 BM and it has square planar structure.
(c) Crystal field splitting energy (Δ0) of [FeF6]4- is higher than the (Δ0) of [Fe(CN)6]4-
(d) crystal field stabilization energy of [V(H2O)6]2+ is higher than the crystal field stabilization of [Ti(H2O)6]2+
Answer:
(d) crystal field stabilization energy of [V(H2O)6]2+ is is higher than the crystal field stabilization of [Ti(H2O)6]2+
II. Answer the following questions
Samacheer Kalvi 12th Chemistry Guide Question 1.
Write the IUPAC names for the following complexes.
- Na2 [Ni(EDTA)]
- [Ag(CN)2]–
- [CO(en)3]2(SO4)3
- [CO(ONO)(NH3)5]2+
- [Pt(NH3)2Cl(NO2)]
Answer:
1. Na2[Ni(EDTA)]
= Sodium EthyicncdiaminetctraacetatonickcEate (II)
(or)
Sodium 2, 2′, 2”, 2” – (ethane – 1, 2 – diyldinitrilo)
tetraacetatonickelate (II)
2. [Ag(CN)2]1
= dicyanidoargentate (I) ion
3. [CO(en)3]2(SO4)3
= tris (ethylenediamine) cobait (III) sulphate
4. [CO(ONO)(NH3)5]2+
= Pentaammincnitrito – kOCobalt (III) ion.
5. [Pt(NH3)2Cl(NO2)]
= diamminedichloridonitrito – kN platinum (II)
12th Chemistry Solutions Samacheer Kalvi Question 2.
Write the formula for the following coordination compounds.
- potassiumhexacyanidoferrate (II)
- petacarbonvliron(O)
- pentaammineriitrito – k – N – cobalt(III)ion
- hexaamminecobalt (III) sulphate
- sodiumtetrafluoridodihydroxidoch romate (III)
Answer:
- potassiurnhexacyanidoferrate (ll) = K4[Fe(CN)6]
- petacarbonyliron(O) = [Fe(CO)5]
- pentaamminenitrito – KN – cobalt (III) ion [CO(NH3)5NO2]2-
- hexaamminecobalt (III) sulphate [CO(CN)6]2(SO4)3
- sodiumtetrafluoridodihyclroxidochromate (III) = Na3[CrF4(OH)2]
Question 3.
Arrange the following in order of increasing molar conductivity
- Mg[Cr(NH3)(Cl)5]
- [Cr(NH3)5Cl]3 [COF6]2
- [Cr(NH3)3Cl3]
Answer:
These complexes can ionise in solution as:
- Mg[Cr(NH3)(Cl)5] = Mg2+ [Cr(NH3) (Cl)5]2-
- [Cr(NH3)5Cl]3 [COF6]2 = [Cr(NH3)5Cl]2+ + [COF6]3-
- [Cr(NH3)3Cl3] = does not ionize
As the number of ions in solution increases, their molar conductivity also increases.
Therefore, conductivity follows the order:
[Cr(NH3)3Cl3] < [Cr(NH3)5Cl]3 [COF6]2 < Mg[Cr(NH3)(Cl)5]
Question 4.
Ni2+ is identified using alcoholic solution of dimethyl glyoxime. Write the structural formula for the rosy red precipitate of a complex formed in the reaction.
Answer:
1. Ni2+ ions present in Nickel chloride solution is estimated accurately for forming an insoluble complex called [Ni(DMG)2] .
2. Nickel ion reacts with alcoholic solution of DMG in the presence of ammonical medium, to give rosy red precipitate of [Ni(DMG)2] complex.
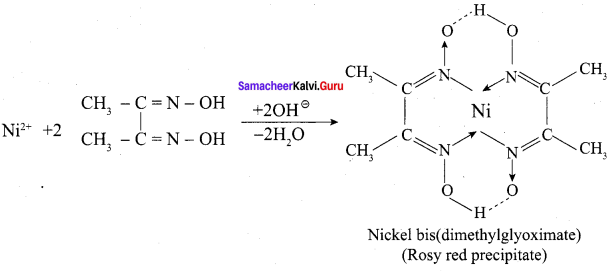
Question 5.
[CuCl4]2- exists while [CuI4]2- does not exist why?
Answer:
1. In [CuI4]2- complex, the size of chloride ion is less hence exist. But in [CuI4]2- the bigger iodide ion makes the compound unstable.
2. When copper cation comes in contact with iodide anion, iodide get oxidised to iodine molecule hence the formation of the above complex ion does not take place. Hence [CuI4]2- exists while [CuI4]2- does not exist.
Question 6.
Calculate the ratio
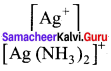
in 0.2 M solution of NH3. If the stability constant for the complex [Ag(NH3)2]+ is 1.7 x 107
Answer:
The stability constant for the complex [Ag(NH3)2]+ is 1.7 x 107, overall dissociation constant is the reciprocal of overall stability constant
K = \(\frac { 1 }{ β }\) ⇒ K =\(\frac { 1 }{ 1.7×107 }\) ⇒ K = 0.588 x 107 ⇒ K = 5.88 x 107
Question 7.
Give an example of coordination compound used in medicine and two examples of biologically important coordination compounds.
Answer:
Medical uses of coordination compounds:
- Ca-EDTA chelate, is used in the treatment of lead and radioactive poisoning. That is for removing lead and radiactive metal ions from the body.
- Cis-platin is used as an antitumor drug in cancer treatment.
Biological important of coordination compounds:
1. A red blood corpuscles (RBC) is composed of heme group, which is Fe2+ Porphyrin complex.it plays an important role in carrying oxygen from lungs to tissues and carbon dioxide from tissues to lungs.
2. Chlorophyll, a green pigment present in green plants and algae, is a coordination complex containing Mg2+ as central metal ion surrounded by a modified Porphyrin ligand called corrin ring. It plays an important role in photosynthesis, by which plants converts CO, and water into carbohydrates and oxygen.
3. Vitamin B12 (cyanocobalamine) is the only vitamin consist of metal ion. it is a coordination complex in which the central metal ion is CO+ surrounded by Porphyrin like ligand.
4. Many enzymes are known to be metal complexes, they regulate biological processes. For example, Carboxypeptidase is a protease enzyme that hydrolytic enzyme important in digestion, contains a zinc ion coordinated to the protein.
Question 8.
Based on VB theory explain why [Cr(NH3)6]3+ is paramagnetic, while [Cr(NH)4]2- is diamagnetic.
Answer:
1. [Cr(NH3)6]3+
In this complex Cr is in the +3 oxidation state. Electronic configuration of Cr atom. Electronic configuration of Cr3+ ion 172 Chemistry 12
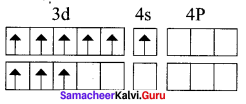
Hybridisation and formation of [Cr(NH3)6]3+ Complex
Due to the presence of three unpaired electrons in
[Cr(NH3)6]3+ it behaves as a paramagnetic substance.
The spin magnetic moment,
µs = \(g\sqrt { 3(3+2) } \) = \(g\sqrt { 15 } \) = 3.87 BM
[Cr(NH3)6]3+ is an inner orbital octahedral complex.
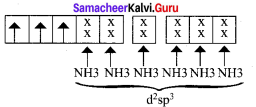
2. [Ni(CN)4]2-
in this complex Ni is in the +2 oxidation state. Electronic configuration of Ni atom. Electronic configuration of Ni2+ ion. Hybridisation and formation of [Ni(CN)4]2- Complex
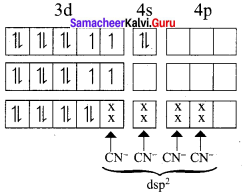
Since CN– is strong field ligand, hence the electrons in 3d orbitais are forced to pair up and there is no unpaired electron in [Ni(CN)4]2 , hence it should be diamagnetic substance.
Question 9.
Draw all possible geometrical isomers of the complex [CO(en)2CI2]+ and identify the optically active isomer.
Answer:
1. Cis – [CO(en)2CI2]+
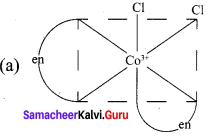
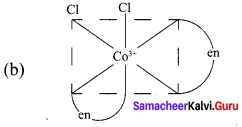
2. Trans [CO(en)2CI2]+
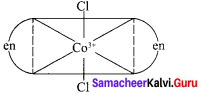
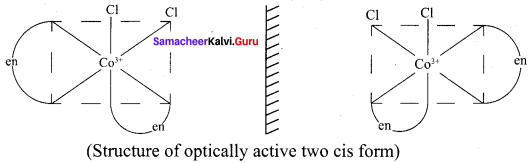
The coordination complex [CO(en)2CI2]+ has three isomers two optically active cis forms and the optically inactive trans form.
Question 10.
[Ti(H2O)6]3+ is coloured, while [Sc(H2O)6]3+ is colourless- explain.
Answer:
Ti in [Ti(H2O)6]3+ is in +3 oxidation state. Sc in [Sc(H2O)6]3+ is in +3 oxidation state. The outer electronic configuration of Sc, Ti and their trivalent ions are,
SC: 3d1 4S2
SC3+: 3d0
Ti: 3d2 4S2
Ti3+: 3d1
Ti3+has one unpaired electron in 3d orbital and they undergoes d-d transition. This electron can be promoted to a higher energy level by light absorption. Therefore [Ti(H2O)6]3+ is coloured. In the case of [Sc(H2O)6]3+ there is no electron in 3d orbital of Sc3+, hence there is no possibility of light absorbance. Therefore [Sc(H2O)6]3+ is colourless.
Question 11.
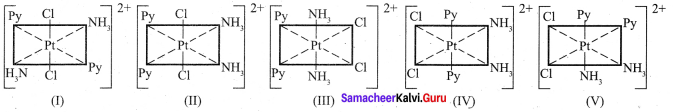
Give an example for complex of the type [Ma2b2c2] where a,b,c are monodentate ligands and give the possible isomers.
Answer:
The octahedral complexes of [Ma2b2c2] type can exist in five geometrical isomers. The five geometrical isomers for the complex ion [PtCl2(NH3)2(py)2]2+are shown below.
Question 12.
Give one test to differentiate [CO(NH3)5Cl] SO4 and [CO(NH3)5SO4] Cl.
Answer:
- [CO(NH3)5Cl]SO4 → [CO(NH3)5Cl]+2 + SO42-
- [CO(NH3)5SO4] Cl → [CO(NH3)5Cl] SO4
1. Aqueous solution of (a) gives sulphate ion. When an addition of BaCL, solution (a) gives white precipitate of BaS04. But (b) does not give any precipitate.
2. Aqueous solution of (b) gives chloride ion. When an addition of AgNO3 solution (b) gives curdy white precipitate of AgCl. But (a) does not give any precipitate.
Question 13.
In an octahedral crystal field, draw the figure to show splitting of d orbitals.
Answer:
Step 1:
In an isolated gaseous state, all the five d orbitals of the central metal ion are degenerate. Initially, the ligands form a spherical field of negative charge around the metal. In this filed, the energies of all the five d orbitals will increase due to the repulsion between the electrons of the metal and the ligand.
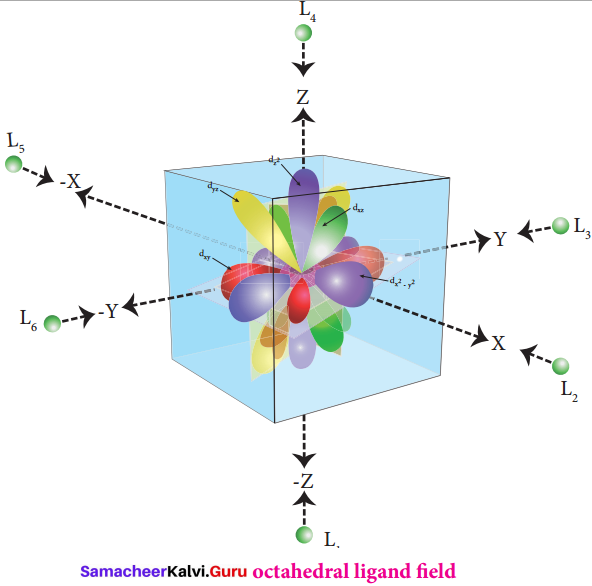
Step 2:
The ligands are approaching the metal atom in actual bond directions. To illustrate this let us consider an octahedral field, in which the central metal ion is located at the origin and the six ligands are coming from the +x, -x, +y, -y, +z and -z directions as shown below. As shown in the figure, the orbitals lying along the axes dx2-y2and dz2 orbitals will experience strong repulsion and raise in energy to a greater extent than the orbitals with lobes directed between the axes (dxy, dyz and dzx). Thus the degenerate d orbitals now split into two sets and the process is called crystal field splitting.
Step 3:
Up to this point the complex formation would not be favoured. However, when the ligands approach further, there will be an attraction between the negatively charged electron and the positively charged metal ion, that results in a net decrease in energy. This decrease in energy is the driving force for the complex formation.
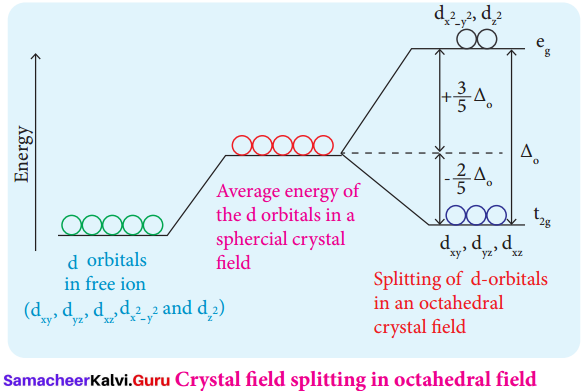
During crystal field splitting in octahedral field, in order to maintain the average energy of the orbitals (barycentre) constant, the energy of the orbitals dx2-y2and dz2 (represented as t2g orbitals) will increase by 3/5∆0 while that of the other three orbitals dxy. dyz and dzx (represented as t2g orbitals) decrease by 2/5∆0 . Here, ∆0 represents the crystal field splitting energy in the octahedral field.
Question 14.
What is linkage isomerism? Explain with an example.
Answer:
This type of isomers arises when an ambidentate ligand is bonded to the central metal atom/ ion through either of its two different donor atoms. For examples – [CO(NH3)5ONO]Cl2 – (Pentaammine nitrito cobalt (III) chloride) – O – attached. (Red in colour).[CO(NH3)5NO2]Cl2 – (Pentaammine nitro cobalt (III) chloride) – N – attached (Yellow-brown in colour).
Question 15.
Write briefly about the applications of coordination compounds in volumetric analysis.
Answer:
Hardness of water is due to the presence of Ca2+and Mg2+ ions in water. EDTA forms stable complexes with Ca2+ and Mg2+. So the total hardness of water can be estimated by simple volumetric titration of water with EDTA.
Question 16.
Classify the following ligand based on the number of donor atoms,
- NH3
- en
- ox2-
- triaminotriethylamine
- pyridine
Answer:
- NH3 – Monodentate ligands (N – Donor atom)
- en – Bidentate ligand (2N – Donor atom)
- ox2- – Bidentate ligand (2O – Donor atom)
- triaminotriethylamine – Tridentate ligand (3N – Donor atom)
- pyridine – Monodentate ligand (N – Donor atom)
Question 17.
Give the difference between double salts and coordination compounds.
Answer:
Double Salt:
- A double salt is’ a compound prepared by the combination of two different salt components.
- Completely dissociate into its ions in water.
- Give simple ions when added to water.
- It can be easily analyzed by determining the ions present in the aqueous solution.
Example : Potash alum K2SO4 . Al2(SO4)3. 24H2O
Coordination compounds (Complex salt):
- A complex salt is a compound composed of a central metal atom having coordination bonds with ligands around it.
- Do not completely dissociate into its ions in water.
- Do not give simple ions.
- It cannot be easily analyzed by determining the ions in the aqueous solution.
Example: Potassium ferro cyanide K4[Fe(CN)6]
Question 18.
Write the postulates of Werner’s theory.
Answer:
1. The central metal ion in any complex ion/compound exhibits two types of valencies, these are (a) Primary valency (b) Secondary valency.
2. The primary valency is ionisable and corresponds to the oxidation state of the metal joining the central ion.
3. The secondary valency is non – ionisable. Every central ion has a fixed number of secondary valencies. This number is called the coordination number of the central ion.
4. The primary valency of the metal ion is always satisfied by a negative ion. The attachment of the central metal ion to the negative ligand is shown by dotted lines.
5. The secondary valencies are satisfied by either negative ions or neutral molecules. The secondary valencies are shown by their lines. The molecules or ions that satisfy secondary valency are called ligands.
6. The ligands which satisfy the secondary valencies must point out in the definite directions in space. Whereas the primary valencies are non – directional in nature. The spatial arrangement of the secondary valencies around the central metal ion is called coordination polyhedron.
7. The secondary valencies are responsible for isomerism in the coordination compounds,
8. Werner’s representation of [CO(NH3)6]Cl3
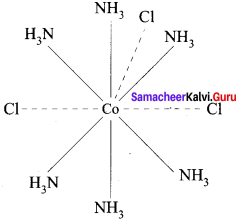
Question 19.
[Ni(CN)4]2- is diamagnetic, while [Ni(CN)4]2- is paramagnetic, explain using crystal field theory.
Answer:
1. [Ni(CN)4]2-
Ni = 3d8 4s2
Ni2+ = 3d8
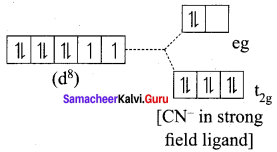
Nature of the complex – Low spin (Spin paired)
Ligand filled elelctronic configuration of central metla ion, t2g6 eg6. Magnetic property – No unpaired electron (CN– is strong filled ligand), hence it is diamagnetic Magnetic moment – µs = 0
2. [Ni(CN)4]2-
Ni = 3d8 4S2
Ni2+ = 3d8
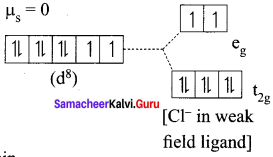
Nature of the complex – high spin
Ligand filled electronic configuration of central metal ion, t2g6 eg6. Magnetic property – Two unpaired electron (CL– is weak field ligand). Hence it is paramagnetic Magnetic moment – it is paramagnetic
Question 20.
Why tetrahedral complexes do not exhibit geometrical isomerism.
Answer:
In tetrahedral geometry
- All the four ligands are adjacent or equidistant to one another.
- The relative positions of donor atoms of ligands attached to the central metal atom are same with respect to each other.
- It has plane of symmetry. Therefore, tetrahedral complexes do not exhibit geometrical isomerism.
Question 21.
Explain optical isomerism in coordination compounds with an example.
Answer:
1. Coordination compounds which possess chairality exhibit optical isomerism similar to organic compounds.
2. The pair of two optically active isomers which are mirror images of each other are called enantiomers.
3. Their solutions rotate the plane of the plane polarised light either clockwise or anticlockwise and the corresponding isomers are called d (dextrorotatory) and 1 (levorotatory) forms respectively.
4. The octahedral complexes of type [M(xx)3]n±, [M(xx)2AB]n± and [M(xx)2B2]n± exhibit optical isomerism.
Eamples:
1. The optical isomers of [Co(en)3]3+ are shown below.
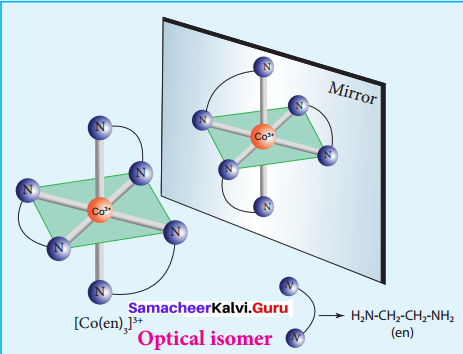
Optical isomer
2. The coordination complex [COCl2(en)2]+ has three isomers, two optically active cis forms and one optically inactive trans form. These structures are shown below.
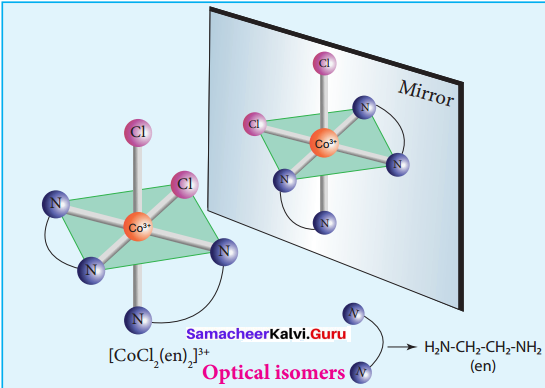
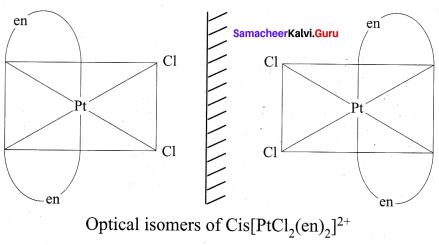
3. In a coordination compound of type [Pt Cl2(en)2]2+, two geometrical isomers are possible. They are cis and trans. Among these two isomers, cis isomer shows optically active isomerism because the whole molecule is asymmetric.
Question 22.
What are hydrate isomers? Explain with an example.
Answer:
The exchange of free solvent molecules such as water, ammonia, alcohol etc., in the crystal lattice with a ligand in the coordination entity will give different isomers. These type of isomers are called solvate isomers. If the solvent molecule is water, then these isomers are called hydrate isomers. For example, the complex with chemical formula CrCl3. 6H2O has three hydrate isomers as shown below.

Question 23.
What is crystal field splitting energy?
Answer:
1. In an octahedral complex, the d – orbitals of the central metal ion divide into two sets of different energies. The separation in energy is the crystal field splitting energy.
2. The d orbitals lying along the axes dx2, dy2 and dz2 orbitals will experience strong repulsion and raise in energy to a greater extent than the orbitals with lobes directed between the axes (dxy, dyz and dzx). Thus the degenerate d – orbitals now split into two sets and the process is called crystal filled splitting.
Question 24.
What is crystal field stabilization energy (CFSE) ?
Answer:
The crystal field stabilisation energy is defined as the energy difference of electronic configurations in the ligand field (ELF) and the isotropic field (Eiso).
CFSE (ΔE0) = {ELF} – {Eiso}
= {nt2g (-0.4) + n6g (0.6) Δ0 – npP} – {n’pP}
Here ntg is the number of electrons in t, orbitals
neg is the number of electrons in e orbitals
np is the number of electrons in the ligand field
n’p is the number of electrons in the isotropic field
Question 25.
A solution of [Ni(H2O)6]2+ is green, whereas a solution of [Ni(CN)4]2- is colorless – Explain.
Answer:
1. In [Ni(H2O)6]2+, Ni is in +2 oxidation state with the configuration 3d8, i.e., it has two unpaired electrons which do not pair up in the presence of weak H2O ligand. Hence, it is coloured. The d – d transtion absorbs red light and the complementary light emitted is green.
2. In the case of [Ni(CN)4]2- Ni is again in +2 oxidation state with the configuration 3d8, but in the presence of strong CN– ligand the two impaired electrons in the 3d orbitals pair up. Thus there is no unpaired electron present. Hence it is colourless. Therefore, a solution of [Ni(H2O)6]2+ is green, whereas a solution of [Ni(CN)4]2- is colourless.
Question 26.
Discuss briefly the nature of bonding in metal carbonyls.
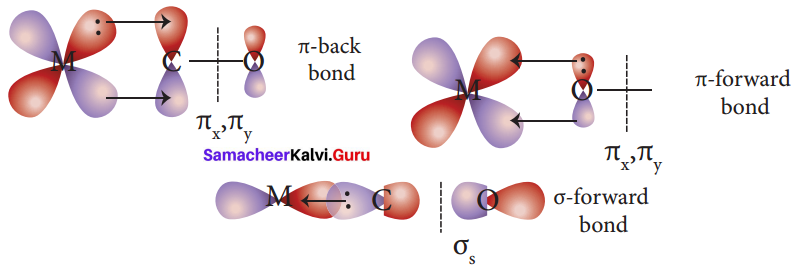
Answer:
1. In metal carbonyls, the bond between metal atom and the carbonyl ligand consists of two components.
2. The first component is an electron pair donation from the carbon atom of carbonyl ligand into a vacant d – orbital of central metal atom. This electron pair donation forms ![]() sigma bond.
sigma bond.
3. This sigma bond formation increases the electron density in metal d- orbitals and makes the metal electron rich.
4. In order to compensate for this increased electron density, a filled metal d-orbital interacts with the empty π* orbital on the carbonyl ligand and transfers the added electron density back to the ligand. This second component is called π – back bonding. Thus in metal carbonyls, electron density moves from ligand to metal through sigma bonding and from metal to ligand through pi bonding, this synergic effect accounts for strong M ← CO bond in metal carbonyls. This phenomenon is shown diagrammatically as follows.
Question 27.
What is the coordination entity formed when excess of liquid ammonia is added to an aqueous solution copper sulphate?
Answer:
When excess of liquid ammonia is added to an aqueous solution of copper sulphate to give tetraamminecopper (II) sulphate

Therefore, the coordination entity is [Cu(NH3)4]2+
Question 28.
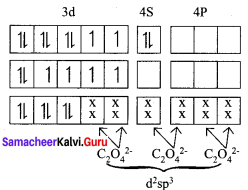
On the basis of VB theory explain the nature of bonding in [CO(C2O4)3]3-.
Answer:
In the complex entity [CO(C2O4)3]3-, the Co is in +3 oxidation state. The outer electronic configuration of CO3+ is 3d6. The oxalato ligand is fairly strong field ligand. So it faces the 3d electrons in CO3+ to pair up and make two of the 3d orbitals available for bonding. As a result, CO3+ shows d2sp2 hybridisation. Electronic configuration of Co atom Electronic configuration of CO3+ ion Hybridisation and formation of [CO(C2O4)3]3-
- There is no unpaired electron in[CO(C2O4)3]3-
Thus[CO(C2O4)3]3- - During the formtion of [CO(C2O4)3]3-, two of the 3d-orbitals are used in bonding. Therefore it is an inner orbital (low spin) complex.
- The [CO(C2O4)3]3- has the octahedral geometry
Question 29.
What are the limitations of VB theory?
Answer:
Limitations of VB – Theory:
1. It does not explain the colour of the complex
2. It considers only the spin only magnetic moments and does not consider the other components of magnetic moments.
3. It does not provide a quantitative explanation as to why certain complexes are inner orbital complexes and the others are outer orbital complexes for the same metal. For example, [Fe(CN)6]4- is diamagnetic (low spin) whereas [Fe(CN)6]4- is paramagnetic (high spin).
Question 30.
Write the oxidation state, coordination number, nature of ligand, magnetic property and electronic configuration in octahedral crystal field for the complex K4[Mn(CN)6].
Answer:
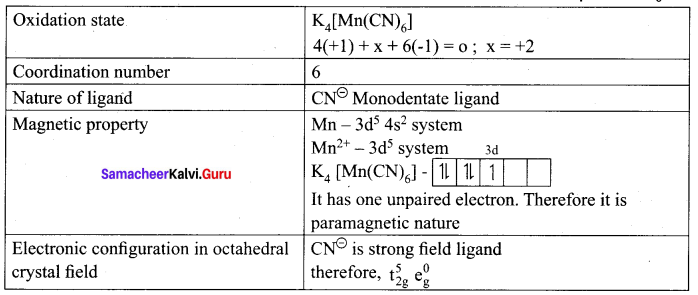
Samacheer Kalvi 12th Chemistry Coordination Chemistry Evaluate Yourself
Question 1.
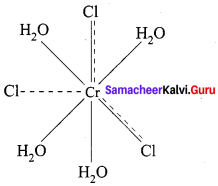
When a coordination compound CrCl3. 4H2O is mixed with silver nitrate solution, one mole of silver chloride is precipitated per mole of the compound. There are no free solvent molecules in that compound. Assign the secondary valence to the metal and write the structural formula of the compound.
Answer:
1. When a coordination compound CrCl3. 4H2O is mixed with silver nitrate solution, one mole of silver chloride is precipitated per mole of the compound. This shows CrCl3. 4H2O complex compound contains one Cl® counter ion.
2. There are no free solvent molecules in CrCl3. 4H2O compound, this shows water molecules are coordinated with central metal ion.
3. Therefore, coordination complex is [CrCl3. 4H2O]Cl Secondary value of the central metal ion is 2Cl® and 4 H2O. Hence coordination number is 6.
4. Werner’s structure of [CrCl2. (H2O)4]Cl
Question 2.
In the complex, [Pt(NO2)(H2O)(NH3)2]Br, identify the following
- Central metal atom/ion
- Ligands(s) and their types
- Coordination entity
- Coordination number
- Oxidation number of the central metal ion
Answer:
[Pt(NO2)(H2O)(NH3)2]Br
- Central metal ion – Pt2+
- Ligands and their types – NO2 – Mono dendata ligand H2O and NH3 – neutral monodendate ligand
- Coordination entity – [pt(NO2(H2O(NH3)2]3+
- Oxidation number of the central metal ion – x + 1(-1) + 1(0) + 1(0) = + 1 ⇒ x – 1 = + 1 ⇒ x = + 2
- Coordination number – 4
Question 3.
Write the IUPAC name for the following compounds.
- K2[Fe(CN)3 (Cl)2 (NH3)]
- [Cr (CN)2 (H2O)] [CO (ox)2 (en)]
- [Cu (NH3)2 Cl2]
- [Cr (NH3)3 (NC)2 (H2O)]+
- [Fe (CN)6]4-
Answer:
- K2[Fe(CN)3 (Cl)2 (NH3)] – Potassium amminedichloridotricyanidoferrate (III)
- [Cr (CN)2 (H2O)] [CO (ox)2 (en)] – Tetraaquadicyanidochromium (II) ethenel,2diaminebis (oxalato) cobalate (II)
- [Cu (NH3)2 Cl2] – diamminedichloro copper (II)
- [Cr (NH3)3 (NC)2 (H2O)]+ – triammineaquodicyanido – KN Chromium (Ill)ion
- [Fe (CN)6]4- – Hexacyanidoferrate (II) ion
Question 4.
Give the structure for the following compounds.
Answer:
- diamminesilver (I) dicyanidoargentate(I)
- Pentaammine nitrito-KNcobalt (III) ion
- hexafluorido cobaltate (III) ion
- dichloridobis(ethylenediamine) Cobalt (III) sulphate
- Tetracarbonylnickel (0)
Answer:
- diamminesilver(I) dicyanidoargentate(I) – [Ag(NH3)9] [Ag(CN)2]
- Pentaammine nitrito-KNcobalt (III) ion – [CO (NH3)5 NO2]+
- hexafluorido cobaltate (III) ion – [COF6]3-
- dichloridobis(ethylenediamine) Cobalt (III) sulphate – [CO(en)2Cl2]SO4
- Tetracarbonylnickel (0) – [Ni (CO)4]
Question 5.
A solution of [CO(NH3)4I2]Cl when treated with AgNO3 gives a white precipitate. What should be the formula of isomer of the dissolved complex that gives yellow precipitate with AgNO3. What are the above isomers called?
Answer:
- A solution of [CO(NH3)4I2]Cl when treated with AgNO3 gives a white precipitate, because Cl-ion is counter ion.
- Formula of isomer of the dissolved complex that gives yellow precipitate with AgNO3 is, [CO (NH3)4 Cl I] I because IΘ is counter ion
- [CO(NH3)4I2]Cl and [ CO(NH3)4 Cl I ] I both are ionisation isomers.
Question 6.
Three compounds A ,B and C have empirical formula CrCl3. 6H2O. they are kept in a container with a dehydrating agent and they lost water and attaining constant weight as shown below.

Answer:
- [Cr(H2O)3 Cl3]. 3H2O
- [Cr(H2O)4 Cl2]Cl. 2H2O
- [Cr(H2O)6]Cl3
Question 7.
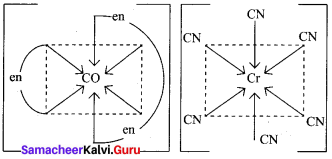
Indicate the possible type of isomerism for the following complexes and draw their isomers
- [CO(en)3][Cr(CN)6]
- [CO(NH3)5(NO2)]2+
- [Pt(NH3)3(NO2)]Cl
Answer:
1. [CO(en)3][Cr(CN)6] – Exhibits coordination isomerism
(a) [CO(en)3][Cr(CN)6]
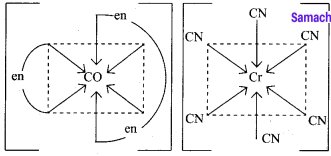
(b) [Cr(en)3][CO(CN)6]
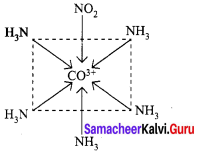
2. [CO(NH3)5(NO2)]2+ – Exhibits linkage isomerism
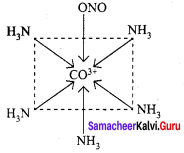
(a) [CO(NH3)5(NO2)]2+ – N attached
(b) [CO(NH3)5(ONO2)]2+ – O attached
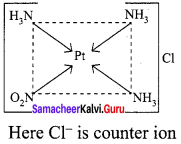
3. [Pt(NH3)3(NO2)]Cl – Exhibits ionisation isomerism
(a) [Pt(NH3)3(NO2)]Cl
(b) [Pt(NH3)3Cl ] NO2
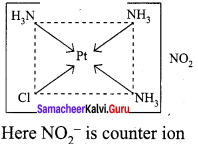
Question 8.
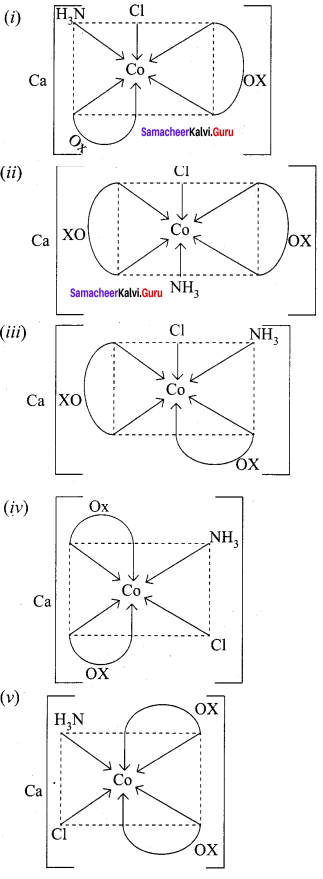
Draw all possible stereo isomers of a complex Ca[CO(NH3)Cl(Ox)2]
Answer:
possible stereo isomers of a complex Ca[CO(NH3)Cl(Ox)2]
Question 9.
The spin only magnetic moment of Tetrachloridomanganate(II)ion is 5.9 BM. On the basis of VBT, predict the type of hybridisation and geometry of the compound. [Mn Cl4]2-
Answer:
Electronic configuration of Mn atom Electronic configuration of Mn2+ ion. Hybridisation and formation of [Mn Cl4]2- complex
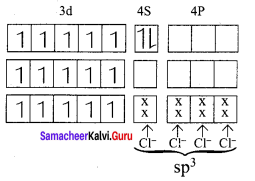
Cl– is weak field ligand no electrons pairing occurs. sp3 hybridisation, It has 5 unpaired electrons. Hence paramagnetic Magnetic moment,
µs = \(g\sqrt { n(n+2) } \)
= \(g\sqrt { 5(5+2) } \)
= \(g\sqrt { 5(7) } \)
= \(g\sqrt { (35) } \)
= 5.9 BM
It has tetrahedral geometry.
Question 10.
Predict the number of unpaired electrons in [COCl4]2- ion on the basis of VBT. [COCl4]2-
Answer:
Electronic configuration of CO atom
Electronic configuration of CO2+ ion
Hybridisation and formation of [COCl4]2- complex
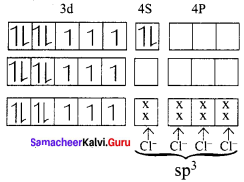
Cl– is weak field ligand, therefore no electrons pairing occurs. sp3 hybridization. It has 3 unpaired electrons, hence it is paramagnetic. Magnetic moment,
µs = \(g\sqrt { n(n+2) }\)
= \(g\sqrt { 3(3+2) } \)
= \(g\sqrt { 15 } \)
= 3.87 BM
It has tetrahedral geometry.
Question 11.
A metal complex having composition CO(en)2Cl2Br has been isolated in two forms A and B. (B) reacted with silver nitrate to give a white precipitate readily soluble in ammonium hydroxide. Whereas A gives a pale yellow precipitate. Write the formula of A and B. state the hybridization of CO in each and calculate their spin only magnetic moment.
Answer:
A metal complex having composition CO(en)2Cl2Br has been isolated in two forms A and B.
1.(B) reacts with silver nitrate to give a white precipitate readily soluble in ammonium hydroxide. This shows (B) has ClΘ counter ion. Hence B is [CO(en)2Cl Br] Cl form.
2. (A) reacts with silver nitrate to give a pale yellow precipitate. This shows (A) has BrΘ counter ion. Hence A is [CO(en)2Cl2] Br.
3. Formula of A and B
- [CO(en)2Cl2] Br
- [CO(en)2Cl Br] Cl
1. A – [CO(en)2Cl2] Br
Electronic configuration of CO atom Electronic configuration of CO3+ atom
Hybridisation and formation of [CO(en)2Cl2] Br complex
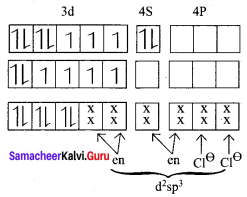
d2 sp3 hybridisation en is strong field ligand. No unpaired electrons, hence it is diamagnetic. Magnetic moment
µs = \(g\sqrt { n(n+2) }\)
n = 0
µs = 0
2. A – [CO(en)2Cl Br] Cl
Electronic configuration of CO atom
Electronic configuration of CO3+ atom
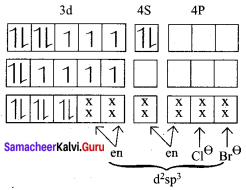
d2 sp3 hybridisation en is strong field ligand. No unpaired electrons, hence it is diamagnetic. Magnetic moment,
µs = 0
∵n = 0
Question 12.
The mean pairing energy and octahedral field splitting energy of [Mn(CN)6]3- are 28,800 cm-1 and 38500 cm-1 respectively. Whether this complex is stable in low spin or high spin?
Answer:
Mean pairing energy = 28,800 cm-1
Octahedral field splitting energy = 38,500 cm-1
[Mn(CN)6]3-
Mn = 3d5 4s2 Mn3+ = 3d4
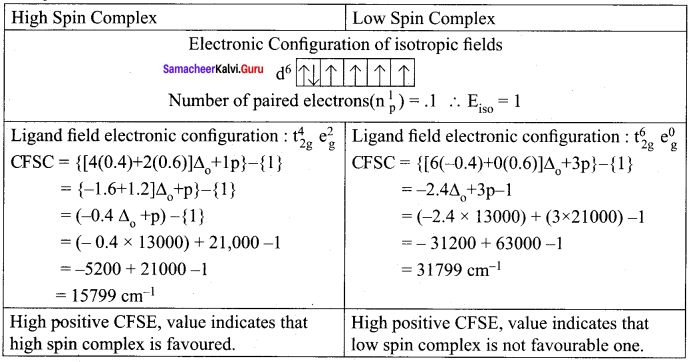
Question 13.
Draw energy level diagram and indicate the number of electrons in each level for the complex [Cu(H2O)6]2+. Whether the complex is paramangnetic or diamagnetic ?
Answer:
Cu in [Cu(H2O)6]2+ has +2 oxidation state.
Electronic configuration of Cu atom – 3d10 4s1
Electronic configuration of Cu2+ ion – 3d9
Distortions in Octahedral Geometry Observed in [Cu(H2O)6]2+
If the ground electronic configuration of a non-linear complex is orbitally degenerate, the complex will distort so as to remove the degeneracy and achieve a lower energy. This is called the Jahn – Effect.
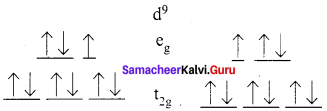
Cu2+ – Two ways of filling the eg orbitals; there is degeneracy and jahn – Teller Distortion is observed
Jahn – Teller Distortion in Cu(III) Complexs [Cu(H2O)6]2+
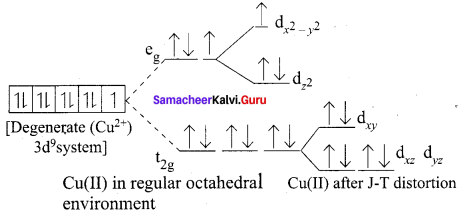
- t2g6 eg3
- It contains one unpaired electron in eg(dx2 – y2 orbital)
- Hence it is paramagnetic
Question 14.
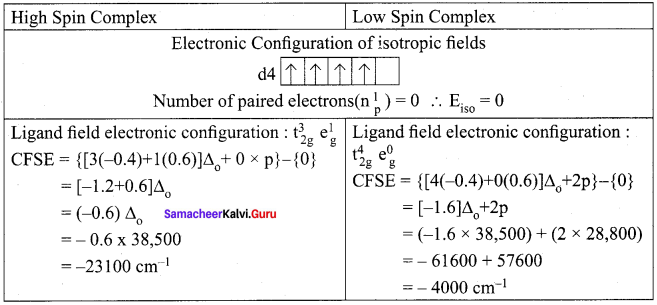
For the [COF6]3- ion the mean pairing energy is found to be 21000 cm-1. The magnitude of ∆0 is 13000 cm-1. Calculate the crystal field stabilization energy for this complex ion corresponding to low spin and high spin states.
Answer:
Mean pairing energy = 21,000cm-1 ∆0 = 13000 cm-1.
[COF6]3-, CO = 3d7 4s2; CO3+ = 3d6
Samacheer Kalvi 12th Chemistry Coordination Chemistry Additional Question
Samacheer Kalvi 12th Chemistry Coordination Chemistry 1 Mark Questions and Answers
I. Choose the correct answer.
Question 1.
Which one of the following is an example of coordination compound?
(a) Common salt
(b) Mohr’s salt
(c) Haemoglobin
(d) Potash alum
Answer:
(c) Haemoglobin
Question 2.
Which one of the following is not an example of complex salt?
(a) Haemoglobin
(b) Chlorophyll
(c) Cobalamine
(d) Ferrous ammonium sulphate
Answer:
(d) Ferrous ammonium sulphate
Question 3.
Which one of the complex salt is acting as a photo sensitiser in photosynthesis process?
(a) Wilkinson’s compound
(b) Cobalamine
(c) Chlorophyll
(d) Haemoglobin
Answer:
(c) Chlorophyll
Question 4.
The complex compound act as oxygen transporter of human is ……………..
(a) Haemoglobin
(b) Chlorophyll
(c) Cyano cobalamine
(d) Wilkinson compound
Answer:
(a) Haemoglobin
5. Which metal is present in vitamin B12?
(a) Iron
(b) Cobalt
(c) Manganese
(d) Copper
Answer:
(b) Cobalt
Question 6.
Which one of the following metal ion is present in Haemoglobin?
(a) Fe2+
(b) CO3+
(c) Mn2+
(d) Cu2+
Answer:
(a) Fe2+
Question 7.
Consider the following statements ……………..
(i) Mohr’s salt answers the presence of Fe2+, NH4+ and SO42- ions.
(ii) Potassium Ferri thio cyanate answers the presence of K+ , Fe3+ , SCN ions
(iii) In coordination compound, the complex ion does not loose its identity and never dissociate to give simple ions.
Which of the above statements is/are correct?
(a) (ii) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii) only
Answer:
(b) (i) and (iii)
Question 8.
How many moles of AgCl are precipitated on the reaction of one mole of COCl3. 5NH3 with AgNO3?
(a) 3
(b) 1
(c) 2
(d) 5
Answer:
(c) 2
Question 9.
What are primary and secondary valency of cobalt in COCl3.6NH3?
(a) 3, 3
(b) 6, 3
(c) 3, 6
(d) 6, 6
Answer:
(c) 3, 6
Question 10.
Consider the following statements.
(i) The outer sphere in coordination compound is called ionisation sphere.
(ii) The primary valences are non directional while secondary valences are directional.
(iii) The primary valances of a metal ion is negative and it is satisfied by positive ions.
Which of the above statements is/are not correct? .
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) only
(d) (ii) only
Answer:
(c) (iii) only
Question 11.
Which one of the following is the coordination entity in k4[Fe (CN)6]?
(a) 4K+
(b) [Fe (CN)6]4
(c) Fe2+
(d) CN–
Answer:
(b) [Fe (CN)6]4-
Question 12.
Which of the following is called Lewis acid in [Ni (CO)4]?
(a) Ni2+
(b) CO
(c) Ni4+
(d) CO
Ans.wer:
(a) Ni2+
Question 13.
Identify the lewis acid in K4[Fe(CN)6]?
(a) Fe3+
(b) Fe2+
(c) K+
(d) CN–
Answer:
(b) Fe2+
Question 14.
The coordination polyhedra of K3 [Fe(CN)6] is ……………..
(a) Square planar
(b) Tetrahedral
(c) Linear
(d) Octahedral
Answer:
(d) Octahedral
Question 15.
The coordination polyhedra of [Ni(CO)4] is ……………..
(a) Octahedral
(b) Tetrahedral
(c) Square planar
(d) Pyramidal
Answer:
(b) Tetrahedral
Question 16.
What is the coordination number of Fe2+ in K4[Fe(CN)6]?
(a) 4
(b) 6
(c) 3
(d) 2
Answer:
(b) 6
Question 17.
Identify the coordination number of Ni2+ in [Ni(en)3]Cl2 ……………..
(a) 3
(b) 2
(c) 6
(d) 5
Answer:
(c) 6
Question 18.
The oxidation state of Fe in [Fe(CN)6]4- is ……………..
(a) II
(b) III
(c) VI
(d) IV
Answer:
(a) II
Question 19.
Identify the oxidation state of cobalt in [CO(NH3)5Cl]2+?
(a) +2
(b) +3
(c) +4
(d) +5
Answer:
(b) +3
Question 20.
What is the coordination number of Pt in [Pt(NO2)(H2O)(NH3)2]Br?
(a) 3
(b) 4
(c) 2
(d) 5
Answer:
(b) 4
Question 21.
Which one of the following is an example of cationic complex?
(a) Na [Ag (CN)2]
(b) [Ag (NH3)2]Cl
(c) [Ni(CO)4]
(d) K4[Fe(CN)6]
Answer:
(b) [Ag (NH3)2]Cl
Question 22.
Which of the following is an example of anionic complex?
(a) [Ag(NH3)2]Cl
(b) [CO (NH3)6]Cl3
(c) [Fe (CO)5]
(d) K4[Fe (CN)6]
Answer:
(d) K4[Fe (CN)6]
Question 23.
Which one of the following is a neutral complex?
(a) [CO (NH3)3 (Cl3)]
(b) [Ag(NH3)2]+
(c) K4[Fe(CN)6]
(d) Na [Ag(CN)2]
Answer:
(a) [CO (NH3)3 (Cl3)]
Question 24.
Which one of the following is a homoleptic complex?
(a) [CO(NH3)3](Cl3)]
(b) [Pt (NH3)2 Cl2]
(c) [Pt(NO2)(H2O)(NH3)2]Br
(d) [Co (NH3)6]Cl3
Answer:
(d) [Co (NH3)6]Cl3
Question 25.
Which one of the following is a heteroleptic complex?
(a) [Pt (NO2) (H2O) (NH3)2]Br
(b) [Ni (CO)4]
(c) [Cb(NH3)6]Cl3
(d) K4[Fe (CN)6]
Answer:
(a) [Pt (NO2) (H2O) (NH3)2]Br
Question 26.
Which one of the following is called as Zeise’s salt?
(а) [Pt (NH3)4] [Pt Cl4]
(b) K[PtCl3(C2H4)]
(c) K4[Fe(CN)6]
(d) [Fe (CO)5]
Answer:
(b) K[PtCl3(C2H4)]
Question 27.
[Pt (NH3)4] [Pt Cl4] is called as ……………..
(a) Zeigler Natta Catalyst
(b) Zeises’ salt
(c) Magnus’s green salt
(d) Mohr’s salt
Answer:
(c) Magnus’s green salt
Question 28.
The IUPAC name of K4[Fe (CN)6] is ……………..
(a) Potassium hexacyanido Ferrate (III)
(b) Potassium hexacyanidoferrate (II)
(c) Potassium ferrocyanide
(d) Potassium ferricyanide
Answer:
(b) Potassium hexa cyanido Ferrate (II)
Question 29.
Which of the following is the IUPAC name of [CO(NH3)6] Cl3?
(a) Hexamminecobalt (III) chloride
(b) Hexammine cobalt (II) chloride
(c) Hexamminechloro cobaltate(III)
(d) Trichlorohexammine cobalt (III)
Answer:
(a) Hexamminecobalt (III) chloride
Question 30.
The IUPAC name of [CO(NH3)4Cl2] Cl is ……………..
(a) Tetrammine dichlorido cobalt (III) chloride
(b) Dichlorido tetrammine cobalt (III) chloride
(c) Tetrammine cobalt (III) trichloride
(d) Tetrammine dichlorido cobaltate (III)
Answer:
(a) Tetramminedichloridocobalt (III) chloride
Question 31.
Which one of the following is the IUPAC name of [Cr (en)3] [CrF6] ……………..
(a) Triethylamine chromium (III) hexa fluriod chromium (III).
(b) Tris (ethane -1, 2 – diamine) chromium (III) hexa flurido chromate (III)
(c) Hexa fluoro chromium (III) tris (ethane – 1, 2 – diamined) chromium (III)
(d) Hexa fluoro chromate (III) triethyl amine chromium (III)
Answer:
(b) Tris (ethane – 1, 2 – diamine) chromium (III) hexa fluorido chromate (III)
Question 32.
The IUPAC name of Na2 [Ni (EDTA)] is ……………..
(a) Disodium tetra acetato nickalate (II)
(b) Sodium 2, 2′, 2″, 2″‘ – (ethane 1,2 – diyldinitrilo) tetra acetato nickelate (II)
(c) Ethylene tetra acetato nickalate (II)
(d) Sodium tetraacetato nickel (II)
Answer:
(b) Sodium 2, 2’, 2″, 2′” – (ethane – 1, 2 – diyldinitrilo) tetra acetato nickelate (II)
Question 33.
The formula of Hexafluorido ferrate (II) ion is ……………..
(a) [Fe F6]4-
(b) [Fe F6]3-
(c) [FeF6]2-
(d) [FeF6]3+
Answer:
(a) [Fe F6]4-
Question 34.
What is the IUPAC name of [CO(CO3) (NH3)4]Cl?
(a) Carbonato tetraammine cobalt (III) chloride
(b) Tetraamminecarbanatocobalt(III) chloride
(c) Carbonato tetra ammonium cobaltate (HI) .
(d) Carbonato tetraammine cobaltate (II)
Answer:
(b) Tetraamminecarbanatocobalt(III) chloride
Question 35.
What is the formula of Diaquadiiododinitrito -k O palladium (IV)?
(a) [Pd I2 (ONO)2 (H2O)2]
(b) [Pd I2 (NO2)2(H2O)2]
(c) [PdI2(NO3)2H2O]
(d) [Pd I2 (NO3) (H2O)]
Answer:
(a) [Pd I2 (ONO)2 (H2O)2]
Question 36.
What is the formula of Triammine triaquachromium (III )chloride?
(a) [Cr Cl3] [Cr (H2O)3]Cl3
(b) [Cr(NH3)3(H2O)3] Cl3
(c) [Cr(H2O)6] [CrCl3]
(d) [Cr (NH3)2 (H2O)4] Cl3
Answer:
(b) [Cr (NH3)3 (H2O)3] Cl3
Question 37.
Which type of isomerism is possible in [CO(NH3)5(NO2)]2+?
(a) Ligand isomerism
(b) Coordination isomerism
(c) Ionisation isomerism
(d) Linkage isomerism
Answer:
(d) Linkage isomerism
Question 38.
[Cr (NH3)4Cl Br]NO, and [Cr (NH3)4Cl NO2] Br are examples of ……………..
(a) Linkage isomerism
(b) Ionisation isomerism
(c) Coordination isomerism
(d) Hydrate isomerism
Answer:
(b) Ionisation isomerism
Question 39.
The type of isomerism present in [Pt(NH3)4] [Pd (Cl)4] and [Pd(NH3)4] [Pt(Cl)4] is ……………..
(a) Solvate isomerism
(b) Ionisation isomerism
(c) Coordination isomerism
(d) Linkage isomerism
Answer:
(c) Coordination isomerism
Question 40.
Isomerism present in CrCl36H2O is ……………..
(a) Solvate isomerism
(b) Ligand isomerism
(c) Linkage isomerism
(d) Ionisation isomerism
Answer:
(a) Solvate isomerism
Question 41.
Geometrical isomerism is exhibited by ……………..
(a) Tetrahedral complex
(b) Linear complex
(c) Square planar complex
(d) All the above
Answer:
(c) Square planar complex
Question 42.
The type of isomerism possessed by [CO (en)3]3+is ……………..
(a) Cis-trans isomerism
(b) Optical isomerism
(c) Ionisation isomerism
(d) Linkage isomerism
Answer:
(A) Optical isomerism
Question 43.
VB theory was proposed by ……………..
(a) Alfred Werner
(b) Bethe and Van vleck
(c) Linus Pauling
(d) Louis de Broglie
Answer:
(c) Linus Pauling
Question 44.
Bethe and Van vleck proposed a coordination theory named as ……………..
(a) Werner’s theory
(b) Valence bond theory
(c) Molecular orbital theory
(d) Crystal field theory
Answer:
(d) Crystal field theory
Question 45.
Which one of the following geometry is possessed by [Cu Cl2]– and [Ag (CN)2] ?
(a) Trigonal planar
(b) Linear
(c) Tetrahedral
(d) Square planar
Answer:
(b) Linear
Question 46.
The type of hybridisation take place in[HgI3]–is ……………..
(a) sp
(b) sp3
(c) sp2
(d) dsp2
Answer:
(c) sp2
Question 47.
Square planar complexes have type of hybridisation ……………..
(a) sp3
(b) dsp2
(c) sp3d
(d) sp3d2
Answer:
(b) dsp2
Question 48.
Which type of hybridisation take place in [Fe(CO)5]?
(a) dsp2
(b) d2sp3
(c) sp3d2
(d) dsp3
Answer:
(d) dsp3
Question 49.
The d orbital involved in dSP3 hybridisation of [Fe (CO)5] is ……………..
(a) dxy
(b) dyz
(c) dxz
(d) dx2 y2
Answer:
(d) dx2 y2
Question 50.
In octahedral geometry, the type of hybridisation involved is ……………..
(a) sp3d2
(b) d2sp3
(c) dsp3
(d) a or b
Answer:
(d) a or b
Question 51.
The d orbitals involved in d2sp3 hybridization are ……………..
(a) dxy, dyz
(b) dx2-y2, dz
(C) dzy, dxz
(d) dxy, dyz
Answer:
(b) dx2-y2, dz
Question 52.
Which type of hybridisation is possible in [Ni(CN)4]2- and [Pt(NH3)4]2+?
(a) dsp2
(b) dsp3
(c) sp3d
(d) sp3d2
Answer:
(a) dsp2
Question 53.
The geometry possible in [Fe F6]4- and [CoF6]4- is ……………..
(a) Trigonal bipyramidal
(b) Square planar
(c) Octahedral
(d) Tetrahedral
Answer:
(c) Octahedral
Question 54.
The geometry of [Fe (CN)6]3- is ……………..
(a) Tetrahedral
(b) Octahedral
(c) Square planar
(d) Trigonamal bipyramidal
Answer:
(b) Octahedral
Question 55.
Which one of the following complex is paramagnetic in nature?
(d) [Ni (CN)4]4-
(b) [Ni(CO)4]
(c) [Fe (CN)6]3-
(d) [Ag(CN)2]–
Answer:
(c) [Fe (CN)6]3-
Question 56.
Which one of the following complex has magnetic moment as 4.899 BM?
(a) [Fe (CN)6]3-
(b) [Ni (CN)4]4-
(C) [COF6]3-
(d) [Ni (CO)4]
Answer:
(c) [CO F6]3-
Question 57.
Consider the following statements,.
(i) VB theory does not explain the colour of the complex
(ii) VB theory does not explain the magnetic properties
(iii) VB theory does not provide a quantitative explanation about inner orbital complexes.
Which of the above statements is/are not correct?
(a) (i) only
(b) (i) and (ii)
(c) (iii) only
(d) (ii) only
Answer:
(c) (iii) only
Question 58.
Consider the following statements,.
(i) Complexes of central metal atom such as of Cu+, Zn2+ are coloured
(ii) Most of the transition metal complexes are colourless
(iii) Negative CFSE value indicates that low spin complex is favoured
Which of the above statements is/are correct?
(a) (i) and (ii)
(b) (iii) only
(c) (ii) only
(d) (i), (ii) only (iii)
Answer:
(b) (iii) only
Question 59.
Which is used for the separation of lanthanides, in softening of hard water and also in removing lead poisoning?
(a) [Ni (CO)4]
(b) EDTA
(c) [Ni(DMG)2]
(d) Ti Cl4 + AI (C2H5)3
Ans.
(b) EDTA
Question 60.
Which complex is used as an antitumor drug in cancer treatment?
(a) Ca – EDTA chelate
(b) EDTA
(c) Ti Cl4 + Al(C2H5)3
(d) Cis – Platin
Answer:
(d) Cis – Platin
Question 61.
What is the name of Na3 [Ag (S2O3)2] ……………..
(a) Sodiumargentothiosulphate
(b) Sodium dithio sulphato angentate (I)
(c) HyPO
(d) Sodium thiosulphate
Answer:
(b) Sodiumdithiosulphatoangentate (I)
Question 62.
Which of the following will give a pair of enantiomorphs?
(a) [Cr (NH3)6 [CO(CN)6]
(b) [CO (en)2 Cl2] Cl
(c) [Pt (NH3)4 [Pt (Cl)6]
(d) [CO (NH3)4 Cl2] NO2
Answer:
(b) [CO (en)2 Cl2] Cl
Question 63.
In which of the following coordination entitites, the magnitude of ∆0 (CFSE in octahedral field) will be maximum?
(a) [CO (CN)6]3-
(b) [CO (C2O4)3]3-
(c) [CO (H2O)6]3+
(d) [CO (NH3)6]3+
Answer:
(a) [CO (CN)6]3-
Question 64.
Which of the following complex ion is expected to absorb visible light?
(a) [Zn (NH3)6]2+
(b) [Sc (H2O)3(NH3)3]3+
(c) [Ti(en)2(NH3)2]4+
(d) [Cr (NH3)6]3+
Answer:
(d) [Cr (NH3)6]3+
Question 65.
Which of the following complex ion is not expected to absorb visible light?
(a) [Ni (H2O)6]3+
(b) [Ni (CN)4]2-
(c) [Cr (NH3)6]3+
(d) [Fe(H2O)6]2+
Answer:
(b) [Ni (CN)4]2-
Question 66.
The IUPAC name of Zeise’s salt is ……………..
(a) Tetramminecopper (II) sulphate
(b) FerrousAmmoniumsulphate
(c) Tetracyanocopper (II) Sulphate
(d) Potassiumtrichloro (ethene) platinate (II)
Answer:
(d) Potassiumtrichloro (ethene) platinate(II)
Question 67.
The CFSE is the highest for ……………..
(a) [CO F4]2-
(b) [CO (NCS)4]2-
(c) [CO (NH3)]3+
(d) [CO Cl4]2-
Answer:
(d) [CO Cl4]2-
Question 68.
Zero magnetic moment will be shown by the compound ……………..
(a) [Cr (NH3)6]3+
(b) [Ag (CN)2]-1
(c) [Fe (CN)6]3-
(d) [COF6]3-
Answer:
(b) [Ag (CN)2]-1
Question 69.
The change of Fe in [Fe (CN)6]3- is ……………..
(a) -6
(b) +3
(c) -3
(d) + 6
Answer:
(A) +3
Question 70.
Coordination number of Co in [CO (F)6]3- is ……………..
(a) 3
(b) 6
(c) 8
(d) 9
Answer:
(b) 6
Question 71.
AgCl precipitate dissolves in ammonium hydroxide due to the formation of ……………..
(a) [Ag (NH4)2] OH
(b) [Ag (NH4)2]C1
(c) [Ag (NH3)2] Cl
(d) [Ag (NH3)2]+1
Answer:
(c) [Ag (NH3)2] Cl
Question 72.
The complexes [CO (NH3)6] [Cr (CN)6] and [Cr (NH3)6] [CO (CN)6] are the example of which type of isomerism?
(a) Linkage isomerism
(b) Ionisation isomerism
(c) Optical isomerism
(d) Coordination isomerism
Answer:
(d) Coordination isomerism
Question 73.
A magnetic moment at 1.73 BM will be shown by one among the following?
(a) Ti Cl4
(b) [Co Cl6]4-
(c) [CU(NH3)4]2+
(d) [N(CN)4]2
Answer:
(c) [CU(NH3)4]2+
Question 74.
Among the following complexes which one shows zero CFSE?
(a) [Mn (H2O)6]3+
(b) [Fe (H2O)6]3+
(c) [CO (H2O)6]2+
(d) [CO (H2O)6]3+
Answer:
(b) [Fe (H2O)6]3+
Question 75.
Number of possible isomers for the complex [CO (en)2 Cl2]Cl will be ……………..
(a) 1
(b) 4
(c) 3
(d) 2
Answer:
(c) 3
Question 76.
The hybridization involved in the complex [Ni (CN)4]2- is ……………..
(a) sp3
(b) d2 sp3+
(c) dsp2
(d) sp3d2
Answer:
(c) dsp2
II. Fill in the blanks:
Question 1.
The reaction between Ferric chloride and potassium thio cyanate solution gives a blood red coloured coordination compound as ……………..
Answer:
K3[Fe (SCN)6 ] Potassium ferrithio cyanate
Question 2.
…………….. is a pigment present in plants acting as a photosensitiser in the photosynthesis.
Answer:
Chlorophyll
Question 3.
In a coordination compound, if the metal ion has a secondary valence of six, it has an …………….. geometry.
Answer:
Octahedral
Question 4.
The coordination polyhedral of [Ni (CO)4] is ……………..
Answer:
Tetrahedral
Question 5.
In [Ni (en)3]Cl2, the coordination number of Fe2+ is ……………..
Answer:
6
Question 6.
In the coordination entity [Fe (CN)6]4-, the oxidation state of iron is represented as ……………..
Answer:
II
Question 7.
The oxidation state of cobalt in [CO (NH3)5Cl]2+ is ……………..
Answer:
+3
Question 8.
The coordination number of Pt in [Pt (NO2) (H2O) (NH3)2] Br is ……………..
Answer:
4
Question 9.
Ethylene diamine tetraacetate has the structure as ……………..
Answer:
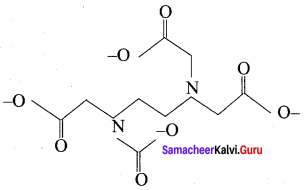
Question 10.
The IUPAC name of k4[Fe (CN)6] is ……………..
Answer:
Potassium hexacyanido – kC ferrate (II)
Question 11.
The complex ion in k4 [Fe (CN)6] is ……………..
Answer:
[Fe (CN)2]4-
Question 12.
The oxidtion state of Fe in k4 [Fe (CN)6] is ……………..
Answer:
II
Question 13.
The coordination number of cobalt in [CO (NH3)4 Cl2]Cl is ……………..
Answer:
6
Question 14.
The IUPAC name of [CO (NH3)4 Cl2]Cl is ……………..
Answer:
Tetra ammine dichlorido cobalt (III) chloride
Question 15.
The IUPAC name of [Cr (en)3] [Cr F6] is ……………..
Answer:
Tris (ethane -1,2- diamine) chromium (III) hexa fluorido chromate (III)
Question 16.
The coordination number of [Cr (en)3] [Cr F6] and oxidation state of Cr are ……………..
Answer:
6, +3
Question 17.
The IUPAC name of [Cr (NH3)3] (H2O)3] Cl3 is ……………..
Answer:
Triamminetriaquachromium(III) chloride
Question 18.
The coordination number of Fe in K3 [Fe (CN)5NO] is ……………..
Answer:
6
Question 19.
The IUPAC name of [Fe F6]4- is ……………..
Answer:
Hexafluriodoferrate(II)ion
Question 20.
The coordination number of cobalt in [CO (NO2)3 (NH3)3] is ……………..
Answer:
6
Question 21.
The IUPAC name of coordination compound [CO (NO2)3 (NH3)3] is ……………..
Answer:
Triamminetrinitrito – K NCobalt (III)
Question 22.
The isomerism possible in [CO (NH3)5 (NO2)]2+ is ……………..
Answer:
Linkage isomerism
Question 23.
The isomerism possible in [Pt(en)2Br2] Cl2 is ……………..
Answer:
Ionisation isomerism
Question 24.
The type of isomerism possible in CrCl3 6H2O is ……………..
Answer:
Solvate isomerism
Question 25.
Geometric isomerism exists in …………….. complexes due to different possible three dimensional spatial arrangement of ligands around the central metal atom.
Answer:
Heteroleptic
Question 26.
…………….. In of the form [MA2B2]n±, cis-trans isomerism exists.
Answer:
Square planar complexes
Question 27.
The square planar complex of the type [M(xy)2n±] shows …………….. isomerism.
Answer:
Geometrical (or) cis-trans
Question 28.
[Pt (NH3)2 Cl2]2+ shows …………….. isomerism.
Answer:
Cis-trans isomerism
Question 29.
[CO Cl2(en)3]3+exhibits …………….. isomerism.
Answer:
Optical isomerism
Question 30.
The hybridised orbitals are and their orientation in space gives a definite …………….. to the complex ion.
Answer:
Directional, Geometry
Question 31.
The shape of [Fe (CO)5] is ……………..
Answer:
Trigonal bipyramidal
Question 32.
The shape of [Ni (CO)4] is whereas the shape of [Ni (CN)4)]2+ is ……………..
Answer:
Tetrahedral, Square planar
Question 33.
The shape of [Hgl3]– is and the type of hybridisation is ……………..
Answer:
Trigonal planar, sp2
Question 34.
The geometry and hybridisation involvedin [CuCl2]– are …………….. respectively.
Answer:
Linear, sp
Question 35.
The hybridisation and geometry of [Fe (CN)6]2- and [Fe (CN)6]3- are …………….. and …………….. respectively
Answer:
d2sp3, octahedral
Question 36.
The shape of [Fe(H2O)6]2+ and [COF6]4-is ……………..
Answer:
Octahedral
Question 37.
The hybridisation take place in [Fe F6]4- and [Fe (H2O)6]2+ is ……………..
Answer:
SP3d2
Question 38.
The d orbital involved in the dsp3 hybridisation of [Fe(CO)5] is ……………..
Answer:
dz2
Question 39.
In the octahedral complexes, if the (n-l)d orbitals are involved in hybridisation, they are called …………….. and ……………..
complexes.
Answer:
The inner orbital complexes, low spin complexes (or) Spin paired complexes.
Question 40.
CO, CN–, en and NH3 are called …………….. ligands.
Answer:
Strong
Question 41.
The magnetic character of [Ni(CO)4] is ……………..
Answer:
Diamagnetic
Question 42.
The hybridisation and geometry of [Ni (CO)4] are …………….. and …………….. respectively.
Answer:
SP3, tetrahedral
Question 43.
The hybridisation and magnetic nature of [Ni (CN)4]2- are …………….. and …………….. respectively.
Answer;
dsp2, diamagnetic
Question 44.
The hybridisation and magnetic nature of [Fe(CN)6]3- are …………….. and …………….. respectively.
Answer:
d2sp3, paramagnetic
Question 45.
The number of unpaired electrons in [Fe (CN)6]3- is and the magnetic moment value is ……………..
Answer:
1,1.73 BM
Question 46.
The hybridisation and geometry of [Co F6]3- are …………….. and …………….. respectively.
Answer:
sp3d2, octahedral
Question 47.
The number of unpaired electrons and magnetic moment value of [Co F6]3- are …………….. and …………….. respectively.
Answer:
4, 4.899 BM
Question 48.
The spin only magnetic moment of tetrachlorido manganate (II) ion is ……………..
Answer:
5.9 BM
Question 49.
[Co (en)2 Cl2]Br react with silver nitrate to form …………….. coloured precipitate.
Answer:
Pale Yellow
Question 50.
The crystal field splitting energy of Ti3+ ion complexes such as [TiBr6]3-, [TiF6]3-, [Ti (H2O)6]3+ the ligands are in the order ……………..
Answer:
Br– < F– < H2O
Question 51.
…………….. is defined as the energy difference of electronic configuration in the ligand field and the isotropic field.
Answer:
Crystal Field stabilisation energy
Question 52.
The hydrated copper (II) ion is in colour as it absorbs …………….. light and transmit its complementary colour.
Answer:
Blue, Orange
Question 53.
The colour of [Ti(H2O)6]3+ is ……………..
Answer:
purple
Question 54.
…………….. is a complex of copper (II) ion used in printing ink and in the packaging industry.
Answer:
Phthalo blue – a bright blue pigment
Question 55.
Purification of Nickel by …………….. process involves formation …………….. which. yields 99.5% pure Nickel on decomposition.
Answer:
Mond’s, [Ni (CO)4]
Question 56.
…………….. is used as a chelating ligand for the separation of lanthanides, in softening of hard water and also in removing poisoning.
Answer:
EDTA, Lead
Question 57.
…………….. process is used in the extraction of silver and gold from their ores.
Answer:
Mac – Arthur – Forrest cyanide
Question 58.
Wilkinson’s catalyst …………….. is used for hydrogenation of alkenes.
Answer:
[(P Ph3)3 Rh Cl]
Question 59.
…………….. is used in the polymerisation of ethane as a complex catalyst
Answer:
Ziegler – Natta catalyst (or) [TiCl4] + Al (C2H5)3
Question 60.
…………….. is used as antitumor drug in cancer treatment.
Answer:
Cis – Platin
Question 61.
In photography, undecomposed AgBr forms a soluble complex called ……………..
Answer:
Sodium dithio sulphato argentate(I)
Question 62.
Ared blood corpuscles (RBC) is composed of heme group which …………….. complex play an important role in carrying oxygen from lungs to tissues.
Answer:
Fe2+ Porphyrin
Question 63.
The green pigment chlorophyll contains …………….. ion surrounded by a modified porphyrin ligand called ……………..
Answer:
Mg2+, corrinring
Question 64.
CO3+ is present in vitamin B12 otherwise chemically called ……………..
Answer:
Cyanocobalamine
Question 65.
The enzyme important in digestion is …………….. contains …………….. coordinated to protein
Answer:
Carboxy peptidase, Zinc ion (Zn2+) Match the following
III. Match the following
Match the List I and List II using the code given below the lsit
Question 1.
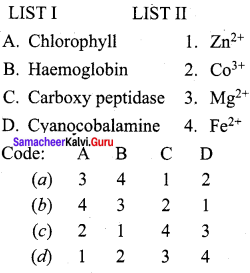
Answer:
(a) 3, 4, 1, 2
Question 2.
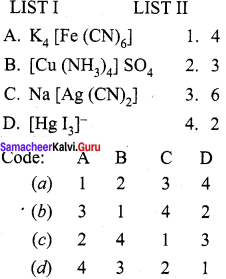
Answer:
(b) 3, 1, 4, 2
Question 3.
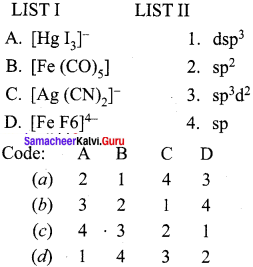
Answer:
(a) 2, 1, 4, 3
Question 4.

Answer:
(c) 3, 4, 2, 1
Question 5.
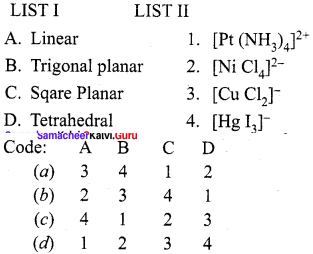
Answer:
(a) 3, 4, 1, 2
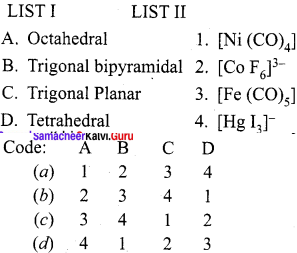
Question 6.
Answer:
(b) 2, 3, 4, 1
Question 7.
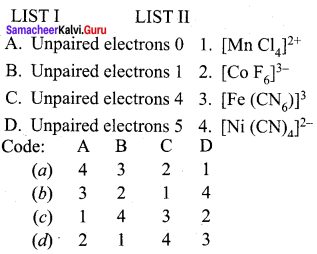
Answer:
(a) 4, 3, 2, 1
Question 8.

Answer:
(a) 2, 3, 4, 1
Question 9.
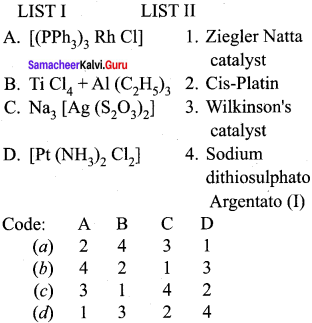
Answer:
(c) 3, 1, 4, 2
Question 10.
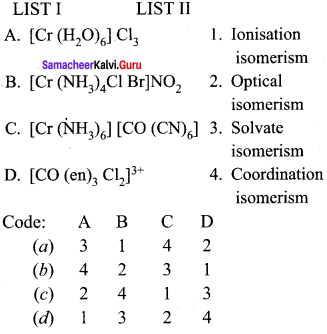
Answer:
(c) 3, 1, 4, 2
IV. Assertion and Reason
Question 1.
ASSertiOfl (A) – Mohr’s Salt answers the presennee of Fe2+, NH42- and SO42- ions.
Reason (R) – The double salt, Mohr’s salt loose their identity and dissociates into their constituent simple ions in solution.
(a) Both A and R arc correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) Both A and R are wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 2.
Assertion (A) – Potassium ferro thiocyanate answers the presences of Fe3+, K+ and SCN– ions.
Reason (R) – The complex ion in coordination compound does not loose its identity and never dissociate to give simple ions.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is wrong but R is correct.
(d) Both A and R are wrong.
Answer:
(c) A is wrong but R is correct.
3. Assertion (A) – The outer sphere in the complex compound is called ionisation sphere.
Reason (R) – The groups prcscnt in outer sphere are loosely bound to the central metal ion and hence can be separated into ions upon dissolving the complex in the suitable solvent.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 4.
Assertion (A) – In K4[Fe (CN)6], the coordination number is six.
Reason (R) – The number of a bonds between ligands and the central metal atom is known as coordination number.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 5.
Assertion (A) – [CO (NH3)6]3 and [Fe (H2O)6]2 are homoleptic complexes
Reason (R) – The central metal ion I atom is coordinated to only one kind of Ligands is called a homoleptic complex.
(a) Both A and R are wrong
(b) A is correct but R wrong
(c) Both A and R are correct and R is the correct explanation of A.
(d) Both A and R are correct and R is not the correct explanation of A.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 6.
Assertion (A) – [CO (NH3)6] [Cr(CN)6] can exist in coordination isomerism.
Reason (R) – In a bimetallic complex, the interchange of one or more ligands between the cationic and the anionic coordination entities result in coordination isomerism
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(e) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 7.
Assertion (A) – [CO(NH3)4Br2]CI and [CO(NH3)4Cl Br] Br are examples of ionisation isomers.
Reason (R) – The exchange of counter ions with one or more ligands in the coordination entity will result in ionisation isomers.
(a) Both A and R are correct and R is not the correct explanation of A.
(b) Both A and R are correct but R is the correct explanation of A.
(c) A and R are wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are correct and R is the correct explanation of A.
Question 8.
Assertion (A) – Geometrical isomerism exists in homoleptic complexes.
Reason (R) – In homoleptic complexes due to different possible three dimensional spatial arrangements of ligands around the central metal atoms.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A and R are wrong.
(d) A is correct but R is wrong
Answer:
(c) A and R are wrong.
Question 9.
Assertion (A) – Geometrical isomerism exists in heteroleptic complexes.
Reason (R) – In heteroleptic complexes due to different possible three dimensional spatial arrangement of ligands around the central metal atom results in geometrical isomers.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) Both A and R are wrong.
(d) A is correct but R is wrong.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 10.
Assertion (A) – [Ni (CO)4] is diamagnetic
Reason (R) – In [Ni (CO)4], there is no unpaired electrons and so it is diamagnetic.
(a) Both A and R are wrong.
(b) A is correct but R is wrong
(c) Both A and R are correct and R is the correct explanaiton of A.
(d) Both A and R are correct but not R is the correct explanaiton of A.
Answer:
(c) Both A and R are correct and R is the correct explanaiton of A.
Question 11.
Assertion (A) – [Fe (CN)6]3- is paramagnetic
Reason (R) – In [Fe (CN)6]3-, there is one unpaired electron and so it is paramagnetic
(a) Both A and R are correct and R is the correct explanation of A,
(b) Both A and R are correct but R is not the correct explanation of A.
(e) Both A and R are wrong.
(d) A is correct but R is wrong.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 12.
Assertion (A) – Most of the transition complexes are coloured.
Reason (R) – Transition complexes absorbs the light of particular wavelength in the visible light. The transmitted light gives the complementary colour.
(a) Both A and R are correct and R is the correct explanation of A.
(b) A is correct but R is wrong.
(e) A and R are wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 13.
Assertion (A) – Complexes of central metal atom such as of Cu+, Zn2+, SC3+, Ti4+ are colourless.
Reason (R) – Cu+, Zn2+, SC3+, Ti4+ are having d0 or d10 configuration and because of it, d – d transition is not possible and so they are colourless.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct and R is not correct explanation of A.
(c) Both A and R arc wrong.
(d) A is correct but R is wrong.
Answer:
(a) Both A and R are correct and R Is the correct explanation of A.
V. Find the Odd one out.
Question 1.
(a) Vitamin – B12
(b) Haemoglobin
(c) Chlorophyll
(d) Glycine
Answer:
(d) Glycine ¡s an amino acid whereas others are complex salts.
Question 2.
(a) Mohr’s salt
(b) Potassium Ferrocyanide
(c) Potassium ferrithio cyanate
(d) Wilkinson’s compound
Answer:
(a) Mohr’s salt is a double salt whereas others are complex salts.
Question 3.
(a) [CO (NH3)6]3+
(b) [Fe (H2O)6]2+
(c) [CO (NH3)3 Cl3]
(d) [Fe (CN)6]+3
Answer:
(e) It is heteroleptic complex whereas others are homoleptic complex.
Question 4.
(a) [CO (NH3)5 Cl]2+
(b) [Pt (NH3)2 CI,]2+
(c) [CO (NH3)6]3+
(d) [Co (NH3)3 CI3]
Answer:
(c) It ¡S homoleptic complex whereas others are heteroleptic complexes.
Question 5.
(a) NH3
(b) CN
(c) H2O
(d) PPh3
Answer:
(b) It is a negative ligand whereas others are neutral ligands
Question 6.
(a) CN–
(b) CI–
(c) SO42-
(d) NH3
Answer:
(d) It is a neutral ligand whereas others are negative ligands.
Question 7.
(a) K4 [Fe (CN)6]
(b) Na [Ag (CN)2]
(c) K2 [Zn (CN)4]
(d [Cu (NH3)4]SO4
Answer:
(d) It is a cationic complex whereas others are anionic complexes.
Question 8.
(a) K3[Fe (CN)6]
(b) [Cu (NH3)4]SO4
(c) [Cr (H2O)] Cl3
(d) [CO(NH3)4CÍ2]Cl
Answer:
(a) It is an anionic complex whereas others are cationic complexes.
Question 9.
(a) [Ti (H2O)6]3+
(b) [Fe (CO)5]
(c) [FeF6]4-
(d) [COF6]4-
Answer:
(b) Fe (CO)I has trigonalbipyramidal shape whereas others have octahedral shape.
Question 10.
(a) [Ag (CN)2]–
(b) [Fe (H2O)6]2+
(c) [Fe F6]4-
(d) [CO F6]4-
Answer:
(a) [Ag (CN)2] is linear in shape whereas others arc in octahedral shape.
VI. Find out the correct pair
Question 1.
(a) [Ni (CO)4], [Ni Cl4]2-
(b) [Cu Cl2], [Fe (CO)5]
(c) [Fe F6]4-, [Fe(CN)6]2-
(d) [Ni (CO)4], [HgI3]–
Answer:
(a) It is tetrahedral whereas others have different shapes.
Question 2.
(a) [Fe (CN)6]3-, [CO F6]3-
(b) [Ni (CN)4]2-, [Ni (CO)5]
(c) [Fe F6], [CO F6]3-
(d) [Cu Cl2], [HgI3]–
Answer:
(a) It is paramagnetic pair whereas others are different.
Question 3.
(a) [Cr (H2O)6] Cl3 and [Cr(H2O)4 Cl2]. 2H2O
(b) [Cr (H2O)5Cl]Cl2. H2O and [Cr (H2O)6]Cl3
(c) [Cr (H2O)4 Cl2]Cl . 2H2O and [Cr(H2O)5 Cl] Cl2. H2O
(d) [Fe (CO)5] and [Ni (CN)4]2
Answer:
(d) [Fe (CO)5] and [Ni (CN)4]2- Others are solvate isomersim.
VII. Find out the incorrect pair
Question 1.
(a) [Fe (F6)]4-, [CO F6]4-
(b) [Cu CI2]–, [Ag (CN)2]–
(c) [Ni (CN)4]2, [Pt (NH3)4]2
(d) [HgI3]–, [Fe (CO)5]
Answer:
(d) [HgI3]–, [Fe (CO)5]
Question 2.
(a) CN– and NO2–
(b) CO and NO
(c) F– and Br
(d) en and (COO–)2
Answer:
(a) CN and NO2. Others are weak ligands.
Samacheer Kalvi 12th Chemistry Coordination Chemistry 2 Marks Questions and Answers
Question 1.
What is the limitations of Werner’s theory ?
Answer:
Werner’s theory was able to explain a number of properties of coordination compounds, it does not explain their colour and magnetic properties.
Question 2.
Differentiate primary valency and secondary valency.
Answer:
Primary Valency:
- The primary valence of a metal ion positive in most of the cases and zero in certain cases.
- The primary valence is always satisfied by negative ions.
- The primary valences are non directional
- Example: In COCl3.6NH3, the primary valence of cobalt is +3
Secondary Valency:
- The secondary valence as the coordination number.
- The secondary valence is satisfied by negative ions, neutral molecular or positive ions.
- The secondary valences are directional
- Example: In COCl3.6NH3, the secondary valence of cobalt is 6
Question 3.
What is coordination entity? Give example.
Answer:
Coordination entity is an ion or a neutral molecule composed of a central action, usually a metal and the array of other groups of atoms (ligands) that are attached to it. For e.g; in potassium ferrocyanide K4[Fe(CN)6] the coordination entity is [Fe(CN)6]4-.
Question 4.
What is meant by central action in complex salt?
Answer:
The central atom / ion is the one that occupies the central position in a coordination entity and binds other atoms or group of atoms (ligands) to itself, through a coordinate covalent bond. For e.g; In K4[Fe(CN)6] the central metal ion is Fe2+.
Question 5.
What are ligands? Give example.
Answer:
The ligands are the atoms or groups of atoms bound to the central metal atom / ion. The atom in a ligand that is bound directly to the central metal atom is known as donor atom. For e.g; In K4[Fe(CN)6] the ligand is CN ion but the donor atom is carbon.
Question 6.
What is meant by coordination sphere? Give example.
Answer:
The complex ion at the coordination compound containing the central metal atom / ion and the ligands attached to it, is collectively called coordination sphere and are usually enclosed in square brackets with the net charge. For e.g; The coordination compound K4[Fe(CN)6] contains the complex ion [Fe (CN)6]4- is referred as coordination sphere.
Question 7.
What is meant by coordination polyhedron?
Answer:
The three dimensional spatial arrangement of ligand molecules / ions that are directly attached to the central metal atom is known as coordination polyhedron (Polygon). For e.g; In K4[Fe(CN)6] the coordination polyhedra is octahedral.
Question 8.
Define coordination number? Give example
Answer:
The number of ligand donor atoms bonded to a central metal ion in a complex is called the coordination number of a metal. In other words, the coordination number is equal to the number of o bonds between ligands and the central metal atom. For e.g; In K4[Fe(CN)6] the coordination number of Fe2+is +6.
Question 9.
What is the coordination number in [Ni (en)3]Cl2? Explain it.
Answer:
In [Ni (en)3]Cl2, the coordination number of Ni2+ is also 6. The ligand “en” represents ethane -1, 2- diamine (H2N – CH2 – CH2 – NH2) and it contains two donor atoms (Nitrogen). Each ligand forms two coordination bonds with nickel. So, totally there are six coordination bonds between them.
Question 10.
Calculate the oxidation number of CO in [CO (NH3)5 Cl]2+.
Answer:
In [CO (NH3)5Cl]2+ let the oxidation number of cobalt is x. The net charge = +2 = x + 5 (0) + 1 (-1)
x – 1 = +2
∴ x = +3
Question 11.
Explain about the types of coordination compound based on kind of ligands?
Answer:
1. A coordination compound in which the central metal ion / atom is coordinated to only one kind of ligands is called homoleptic complex, e.g; [CO (NH3)6 ]3+
2. The central metal ion/atom is coordinated to more than one kind of ligands is called a heteroleptic complex, e.g; [CO (NH3)5 Cl]2+
Question 12.
Write the IUPAC names of the following complex salts.
- [CO (NH3)6]Cl3
- K4 [Fe (CN)6]
Answer:
- [CO (NH3)6]Cl3 – Hexaamminecobalt (III) Chloride
- K4 [Fe (CN)6] – Potassiumhexacyanido – K C ferrate (II)
Question 13.
Give the formula and IUPAC name of the following ligands.
(i) OX
(ii) en
Answer:
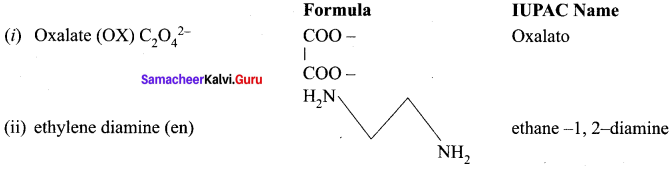
Question 14.
Give the formula of
- EDTA
- Triphenyl phosphine
Answer:
1. EDTA
2, 2′, 2″, 2″‘ – (ethane – 1, 2 – diyldinitrilo ) tetraacetato
2. Triphenyl phosphine – P (Ph)
Question 15.
Give the IUPAC names of the following compounds.
- [PdI2(ONO)2 (H2O)2]
- [Cr (PPh3) (CO)5]
Answer:
- [Pd I2 (ONO)2 (H2O)2] – Diaquadiiododinitrito – kopalladium (iv)
- [Cr (PPh3) (CO)5] – Pentacarbonyltriphenylphosphanechromium (O)
Question 16.
Give the IUPAC names of the following compounds.
- [CO (NO2)3 (NH3)3]
- K3 [Fe (CN)5NO]
Answer:
- [CO (NO2)3 (NH3)3] – Triamminetrinitrioto – KN Cobalt (III)
- K3 [Fe (CN)5NO] – Potassiumpentacyanidonitrosylferrate (II)
Question 17.
Give the IUPAC names of the following compounds.
- Na2[Ni (EDTA) ]
- [CO (NH3)5Cl]2+
Answer:
- Na2[Ni (EDTA) ] – Sodium 2,2′,2″,2″ – (ethane -1, 2- diyldinitrilo) tetraacetato nickelate (II)
- [CO (NH3)5Cl]2+ – Pentaamine chloro cobalt (III) ion
Question 18.
Give the IUPAC names of the following compounds.
- [Ag (NH3)2]+
- [FeF6]4-
Answer:
- [Ag (NH3)2]+ – Diammine silver (I) ion
- [FeF6]4- – Hexa fluoro ferrate (II) ion.
Question 19.
Define isomerism in coordination compounds.
Answer:
Isomerism is the phenomenon in which more than one coordination compounds having the same molecular formula have different physical and chemical properties due to different arrangement of ligands around the central metal atom.
Question 20.
What are the different types of isomerism in coordination compounds?
Answer:
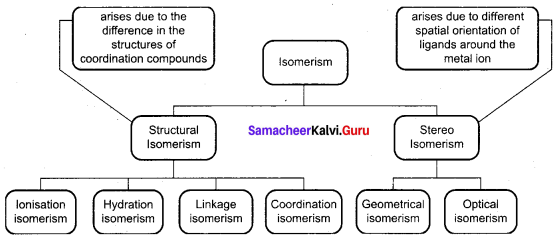
Question 21.
Define stereo isomerism in coordination compound. Give its type.
Answer:
The phenomenon in which the coordination compounds have the same chemical formula and connectivity between the central metal atom and the ligands is known as stereo isomerism. But they differ in the spatial arrangement of ligands in three dimensional space.They can be further classified as (a) Geometrical isomerism (b) Optical isomerism
Question 22.
Define crystal field stabilisation energy. (CFSE).
Answer:
It is defined as the energy difference of electronic configurations in the ligand field (ELF) and the isotropic field / barycentre (Eiso)
CFSE (ΔE0) = {ELF} – {Eiso}
= {[nt2g – (0.4) + neg (0.6)] ∆0 +npP] – n’pP}
Where,
nt2g = the number of electrons in t,„ orbital.
neg = the number of electrons in n orbital
np = number of electron pairs in the ligand field.
n’p = number of electron pairs in the iso tropic field.
Question 23.
What are metallic carbonyl? Give example.
Answer:
Metal carbonyls are the transition metal complexes of carbon monoxide, containing metal carbon bond. In these complexes CO molecule act as a neutral ligand, e.g – [Ni (CO)4] Nickel tetra carbonyl, a homoleptic complex.
Samacheer Kalvi 12th Chemistry Coordination Chemistry 3 Marks Questions and Answers
Question 1.
Explain Werner’s postulate using CO Cl3. 6NH3.
Answer:
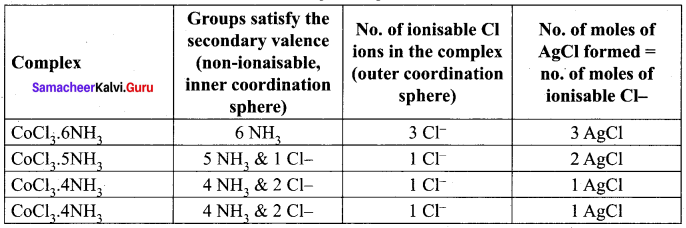
Question 2.
What is the oxidation state in coordination compound? Explain with example.
Answer:
The oxidation state of a central metal atom in a coordination entity is defined as the charge it would bear if all the ligands were removed along with the electron pairs that were shared with the central atom. In naming a complex, it is represented by a Roman numeral. For e.g., in coordination entity [Fe (CN)6]4- the oxidation state of iron is represented as (II). In [Fe (CN)6]4- , let the oxidation number of iron is x
The net charge = – 4
x + 6 (-1) = – 4
x – 6 = – 4
x = 6 – 4
= +2
So, iron oxidation state (II).
Question 3.
Explain the types of complexes based on the charge on the complex.
Answer:
The coordination compounds can be classified into the following types based on the net charge of the complex ion. A coordination compound in which the complex ion
- carries a net positive charge is called a cationic complex.
Example [Ag (NH3)2]+ - carries a net negative charge is called an anionic complex.
Example [Ag (CN)2]– - bears no net charge is called a neutral complex.
Example [Ni (CO)4]
Question 4.
What is meant by ligand? Explain their types with examples.
Answer:
The ligands are the atoms of groups of atoms bound to the central metal atom / ion. The atom in a ligand that is bound directly to the central metal atom is known as a donor atom.
Ligands are of 5 types
- Cationic ligand – e.g., NH2 – NH3+ Hydrazinium
- Anionic ligand – e.g., Br Bromido
- Neutral ligand – e.g., H2O Aqua
- Mono dendate ligand – A ligand can be connected by one coordinate bond, e.g – F fluorido
- Ambidendate ligand – A ligand can be connected by more than one coordinate mode.
- e.g., – NCS iso thio cyanato (KN)
- – SCN thio cyanato (KS)
Question 5.
Identify the following terms in the complex [K4Fe (CN)6]
(i) Cation
(ii) Anion
(iii) ligands
(iv) central metal ion
(v) Oxidation state of metal
(vi) IUPAC name
Answer:
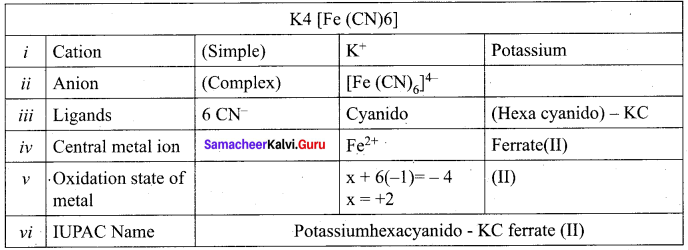
Question 6.
Identify and write the following in the complex [CO (NH3)4 Cl2]Cl
(i) Cation
(ii) Ligands
(iii) Name of the ligand
(iv) central metal
(v) Oxidation state of central metal
(vi) Anion
(vii) IUPAC name [CO (NH3)4 Cl2]Cl
Answer:
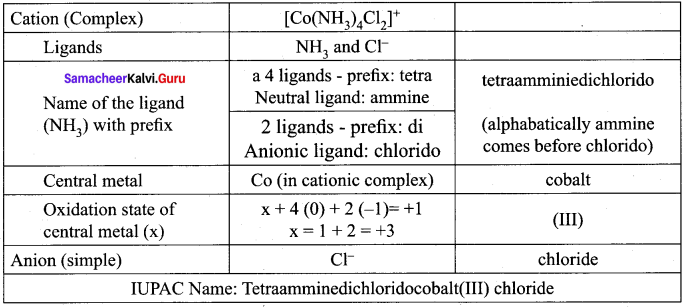
Question 7.
Write the following in the complex [Cr (en)3] [Cr F6]
- Type of complex
- Ligands
- central metal
- Oxidation state of central metal
- IUPAC name
Answer:
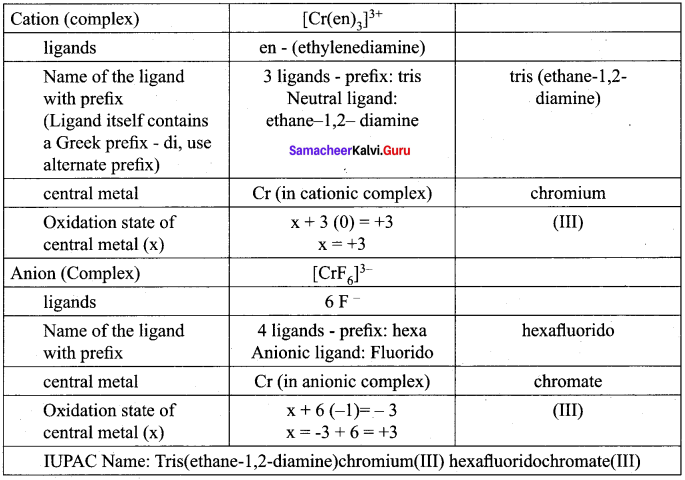
Question 8.
Write the IUPAC names of the following complexes.
- [CO (NH3)5 CN] [CO (NH3) (CN)5]
- [Pt (Py)4 ] [Pt Cl4]
- [CO (NH3)4 Cl2]3 [Cr(CN)6]
Answer:
1. [CO (NH3)5 CN] [CO (NH3) (CN)5]
Penta ammine cyanido – K C Cobalt (III) ammine penta cyanido – K C Cobaltate (III)
2. [Pt (Py)4 ] [Pt Cl4]
Tetra pyridine platinum (II) tetrachlorido platinate (II)
3. [CO (NH3)4 Cl2]3 [Cr(CN)6]
Tetraammine dichlorido cobalt (III) hexacyanido KC chromate (III)
Question 9.
Explain coordination isomerism with suitable example.
Answer:
1. Coordination isomerism arises in the coordination compounds having both the cation and anion as complex ions. The interchange of one or more ligands between cationic and the anionic coordination entities result in different isomers.
2. Fox e.g., in the coordination compound, [CO (NH3)6] [Cr (CN)6], the ligands ammonia and cyanide were bound respectively to cobalt and chromium while in its coordination isomer [Cr (NH3)6] [CO (CN)6], they are reversed.
Question 10.
Explain ionisation isomerism with suitable example.
Answer:
1. Ionisation isomerism arises when an ionisable counter ion (simple ion) itself can act as a ligand.
2. The exchange of such counter ions with one or more ligands in the coordinatioin entity will result in ionisation isomers. These isomers will give different ions in solution.
3. For example, consider the coordination compound [Pt (en)2 Cl2]Br2. In this compound, both Br and CP have the ability to act as a ligand and the exchange of these two ions result in a different isomer [Pt (en)–, Br2]Cl,. In solution, the first compound Br– ions while the later gives CP ions and hence these compounds are called ionisation isomers.
Question 11.
Explain the type of isomers possible for the formula [Cr (H2O)6]Cl3 with their colour.
Answer:
- Cr Cl3 6H2O has three hydrate isomers.
- [Cr (H2O)6]Cl3 – A violet colour compound and gives 3 chloride ions in solution.
- [Cr (H2O)5]Cl2 H2O – A pale green colour compound and gives 2 chloride ions in solution.
- [Cr (H2O)4 Cl2]Cl.2H2O – A dark green colour compound and gives one chloride ion in solution.
Question 12.
Mention the coordination number, hybridisation and geometry of the following compounds.
(i) [Cu Cl2]–
(ii) [Hg I3]–
(iii) Ni (CO)4
Answer:

Question 13.
Metion the coordination number, hybridisation and geometry of the following compounds.
(i) [Ni(CN)4]2-
(ii) [Fe (CO)5]
(iii) [CO(NH3)6]3+
Answer:

Question 14.
How is spectrochemical series is used to identify the type of ligands?
Answer:
1. I– < Br– < SCN– < Cl < S2- < F– < OH– ≈ urea < OH2- < H2O < NCS– < EDTA4- < NH3 < en < NO2– < CN < CO The above series is known as spectrochemical series.
2. The ligands present on the right side of the series such as carbonyl causes relatively larger crystal field splitting and are called strong ligands (or) strong field ligands.
3. The ligands on the left side are called weak field ligands and causes relatively smaller crystal field splitting.
Question 15.
Most of the transition metal complexes are coloured. Justify this statement.
Answer:
1. A substance exhibits colour when it absorbs the light of a particular wave length in the visible region and transmit the rest of the visible light.
2. When this transmitted light enters our eye, our brain recognises its colour. The colour of the transmitted light is given by the complementary colour of the absorbed light.
3. For e.g., the hydrate copper (II) ion is blue in colour as it absorbs orange light and transmit its complementary colour, blue.
Question 16.
Using crystal field theory, explain the colour of the coordination compound.
Answer:
- The ligand field causes the splitting of d orbitals of the central metal atom into two sets (t2g and eg).
- When the white light falls on the complex ion, the central metal ion absorbs visible light corresponding to the crystal field splitting energy and transmits rest of the light which is responsible for the colour of the complex.
- This absorption causes excitation of d – electrons of the central metal ion from the lower energy t2g level to the higher energy eg level which is known as d – d transition.
Question 17.
Ti (H2O)6]2+ is purple in colour. Prove this statement.
Answer:
- In [Ti (H2O)6]2+, the central metal ion is Ti3+ which has d1 configuration. This single electron occupies one of the t2g orbitals in the octahedral aqua ligand field.
- When white light falls on this complex, the d electron absorbs light and promotes itself to eg level.
- The spectral data show the absorption maximum is at 20000 mol-1 corresponding to the crystal field splitting energy (∆0) 239.7 kJ mol-1. The transmitted colour associated with this absorption is purple and hence the complex appears in purple in colour.
Question 18.
Cu+, Zn2+, Sc3+, Ti4+ are colourless. Prove this statement.
Answer:
- Cu+, Zn2+ have d10 configuration and SC3+, Ti4+ have d1 configuration.
- d – d transition is not possible in the above complexes. So they are colourless.
Question 19.
Explain about the bonding in metal carbonyls.
Answer:
1. In metal carbonyls, the bond between metal atom and the carbonyl ligand consists of two components. The first component is an electron pair donation from the carbon atom of the carbonyl ligand into a vacant d – orbital of central metal atom. This electron pair donation forms M \(\underleftarrow { \sigma bond }\) CO and sigma bond.
2. This a bond formation increases the electron density in metal d orbitals and makes the metal electron rich.
3. In order to compensate for this increased electron density, a filled metal d – orbital interacts with the empty n orbital on the carbonyl ligand and transfers the added electron density back to the ligand. This second component is called n back bonding.
4. Thus in metal, carbonyl, electron density moves from ligand to metal through o bonding and from metal to ligand through Pi bonding, this synergic effect accounts for strong M ← CO bond in metal carbonyls.
Question 20.
Explain the medicinal applications of coordination compounds?
Answer:
- Ca – EDTA chelate is used in the treatment of lead and radioactive poisoning. This is for removing lead and radioactive metal ions from the body.
- Cis – platin is used as an anti tumor drug in cancer treatment
Samacheer Kalvi 12th Chemistry Coordination Chemistry 5 Marks Questions and Answers
Question 1.
Write the IUPAC name of the following complexes.
- [Ag(NH3)2]Cl
- [CO (en)2 Cl2]CI
- [Cu (NH3)4]SO4
- [CO (CO3) (NH3)4] Cl
- [Cr(NH3)3(H2O)3]Cl3
Answer:
- [Ag(NH3)2]Cl – Diammine Silver (I) Chloride
- [CO (en)2 Cl2]CI – Dichloridobis (ethane -1, 2- diamine) Cobalt (III) chloride.
- [Cu (NH3)4]SO4 – Tetraammine copper (II) Sulphate
- [CO (CO3) (NH3)4] Cl – Tetrammine carbanato cobalt (III) chloride
- [Cr (NH3)3 (H2O)3]Cl3 – Triammine tri aqua chromium (III) chloride
Question 2.
Explain about the geometrical isomerism in complexes having coordination number 4.
Answer:
1. Geometrical isomerism exists in heteroleptic complexes due to different possible three diamensional spatial arrangements of the ligands around the central metal atom. This type of isomerism exist in square planar tetrahedral complexes.
2. In square planar complexes of the form [M A2 B2 ]n± and [MA2BC]N+ where A, B and C are monodentate ligands and M is the central metal ion / atom.
3. Similar groups (A or B) present either on same side or on the opposite side of the central metal atom (M) give rise to two different geometrial isomers and they are called cis and trans isomers respectively.
4. The square planar complex of the type [M (XY)2]n± where XY is a bidentate ligand with two different coordinating atom also shows cis-trans isomerism.
5. Square planar complex of the form [MABCD]n± also shows cis – trans isomerism. In this case, by considering any one of the ligands [A,B,C,D] as a reference, the rest of the ligands can be arranged in three different ways leading to three geometrical isomers.
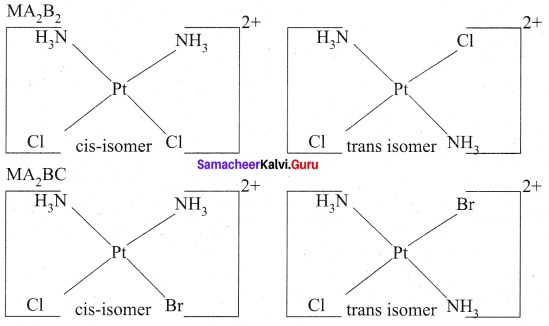
Question 3.
Explain about the geometrical isomerism of octahedral complexes with suitable example.
Answer:
1. Octahedral complexes of the type [M A2 B2]n± , [M (xx)2 B2]n± shows cis-trans isomerism. Hence A and B are monodentate ligands and xx is bidentate ligand with two same kind of donor atoms. In the octahedral complex, the position of ligands is indicated by the. following numbering scheme.
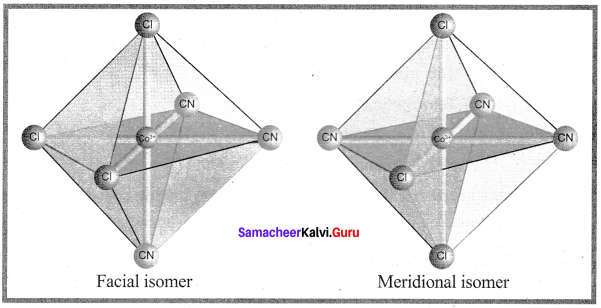
2. The positions (1,2) (1,3) (1,4) (1,5), (2,3) (2,5) (2,6), (3,4) (3,6) (4,5) (4,6) and (5,6) are identical and if two similar groups are present in any one of these positions, the isomer is referred as a cis-isomer.
3. Similarly positions (1,6), (2,4) and (3,5) are identical and if similar groups (or) ligands are present in these positions it is referred as a trans – isomer.
4. Octahedral complex of the type [MA3B3]n± also shows geometrical isomerism. If the three similar ligands (A) are present in the comers of one triangular face of the octahedron and the other 3 ligands (B) are present in the opposing triangular face, then the isomer is referred as a facial isomer (fac isomer).
5. If the three similar ligands are present around the meridian which is an imaginary semicircle from one apex of the octahedral to the opposite apex, the isomer is called a meridional isomer (mer is omer). This is called a meridional because each set of ligands can be regarded as lying on a meridian of an octahedran.
Question 4.
Describe about the postulate of VB theory (or) Valence bond theory.
Answer:
1. The ligand → metal bond in a coordination complex is covalent in nature. It is formed by the sharing of electrons between the central metal atom and electron donor ligand.
2. Each ligand should have atleast one filled orbital containing a lone pair of electrons.
3. In order to accommodate the electron pairs donated by the ligands, the central metal ion present in a complex provides required number of vacant orbitals.
4. These vacant orbitals of central metal atoms undergo hybridisation, the process of mixing of atomic orbitals of comparable energy to form equal number of new orbitals called hybridised orbitals with same energy.
5. The vacant hybridised orbitals of the central metal ion, linearly overlap with filled orbitals of the ligands to form coordinate covalent sigma bonds between the metal and the ligand.
6. The hybridised orbitals are directional and their orientation in space gives a definite geometry to the complex ion.
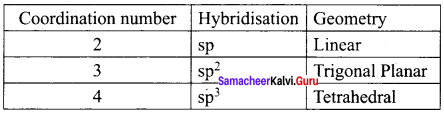
7. In the octahedral complexes, if the (n-l)d orbitals are involved in hybridisation they are called inner orbital complexes or low spin complexes (or) spin paired complexes. If the nd orbitals are involved in hybridisation, such complexes are called outer orbital complexes (or) high spin (or) spin free complexes. Here “n” represents the principal quantum number of the outermost shell.
8. The complexes containing a central metal atom with unpaired electron (s) are paramagnetic. If all the electrons are paired, then the complexes will be diamagnetic.
9. Ligands such as CO, CN–, en and NH3 present in the complexes cause pairing of electrons present in the central metal atom. Such ligands are called strong field ligands.
10. Greater the overlapping between, the ligand orbitals and the hybridised metal orbital, greater is the bond strength.
Question 5.
Explain the hybridisation, magnetic property, geometry, magnetic moment of [Ni (CO)4] Using valence bond theory
Answer:
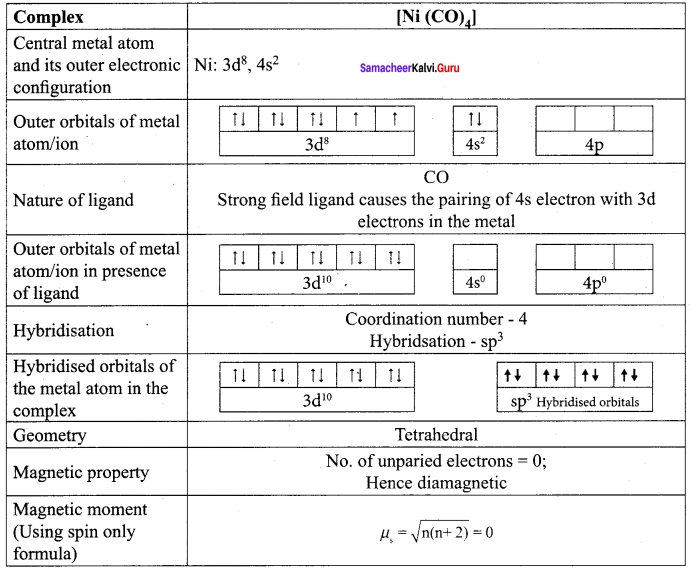
Question 6.
Explain the hybridisation, geometry, magnetic property and magnetic moment of [Ni (CN)4]2- using valence bond theory.
Answer:
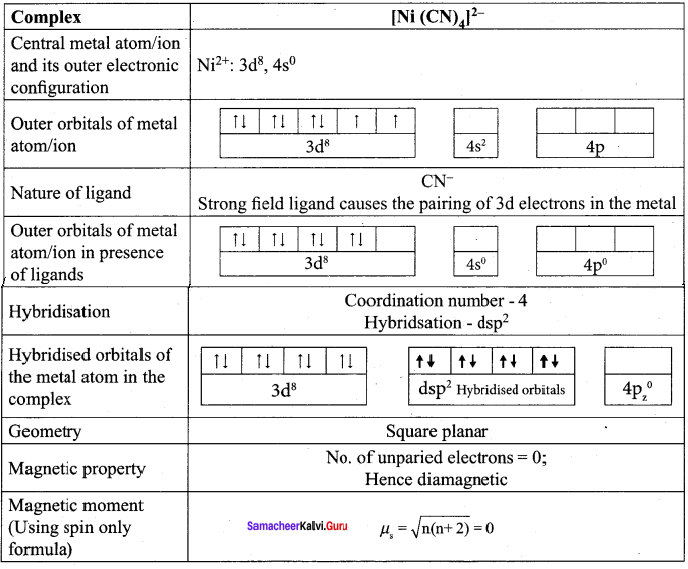
Question 7.
Using VB theory, explain the type of hybridisation, geometry, magnetic property and magnetic moment of [Fe (CN)6]3-.
Answer:
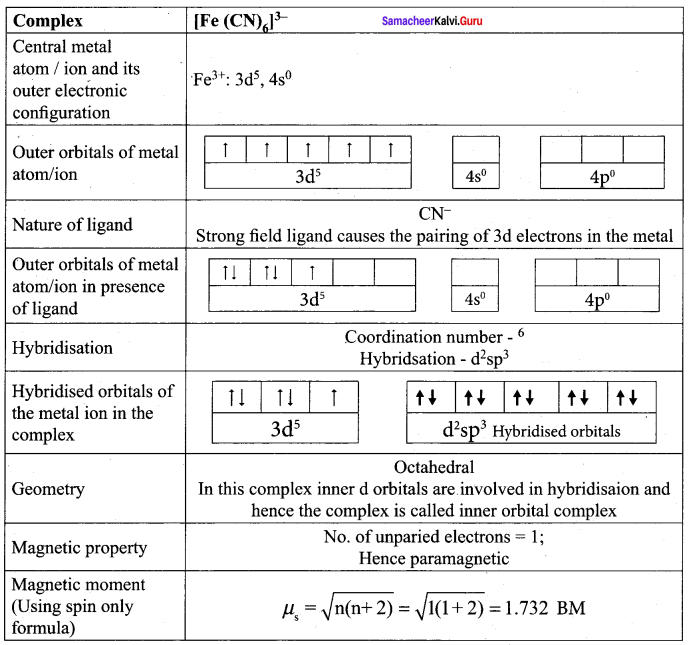
Question 8.
Explain the hybridisation, geometry, magnetic property and magnetic moment [CO F6]3-
Answer:
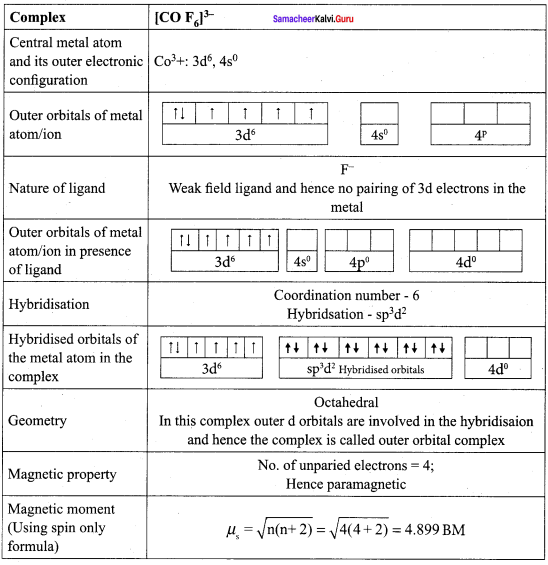
Question 9.
Explain about crystal field theory.
Answer:
- Crystal field theory assumes that the bond between the ligand and the central metal atom is purely ionic, i.e., the bond is framed due to the electrostatic attraction between the electron rich ligand and electron deficient metal.
- In the coordination compounds, the central metal atom/ion and the ligands are considered as point charges (or) electric dipoles.
- cording to crystal field theory, the complex formation is considered as the following series of hypothetical steps.
Step 2:
The ligands are approaching the metal atom in actual bond directions. Consider an octahedral field, in which the central metal ion is located at the origin and six ligands are coming from the +x, -x, +y, -y , +z and -z directions. The orbitals lying along the axes dx2 – y2 and dz2orbitals will experience strong repulsion and raise in energy to a greater extent than the orbitals with lobes directed between the axes (dxy, dyz and dxz). Thus the degenerate d orbitals now split into two sets and the process is called crystal field splitting.
Step 3:
Upto this point the complex formation would not be favoured. However when the ligands approach further, there will be an attraction between the negatively charged electron and the positively charged metal ion that results in a net decrease in energy. This decrease in energy is the driving force for the complex formation.
Question 10.
Describe about the crystal field splitting in tetrahedral complex.
Answer:
1. Consider a cube in which the central metal atom is placed at its centre (i.e., origin of the coordinate axis). The four ligands approach the central metal atom along the direction of the leading diagonals from the alternate comers of the cube.
2. In this field, the t2g orbitals (dxy, dyz and dzx) are pointing close to the direction in which ligands are approaching than the ‘eg’ orbitals. (dx2 – y2 and dz2). As a result, the energy of t2g orbitals increases by 2/5 ∆t and that of ‘e’ orbitals decreases by 3/5 ∆t as shown in the figure. This splitting is inverted when compared to octahedral field.
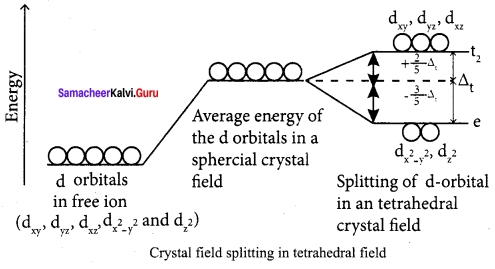
Question 11.
How would you calculate crystal field stabilization energy (CBSE) for [Fe (H2O)6]3+. Complex: [Fe(H2O)6]3+
Answer:
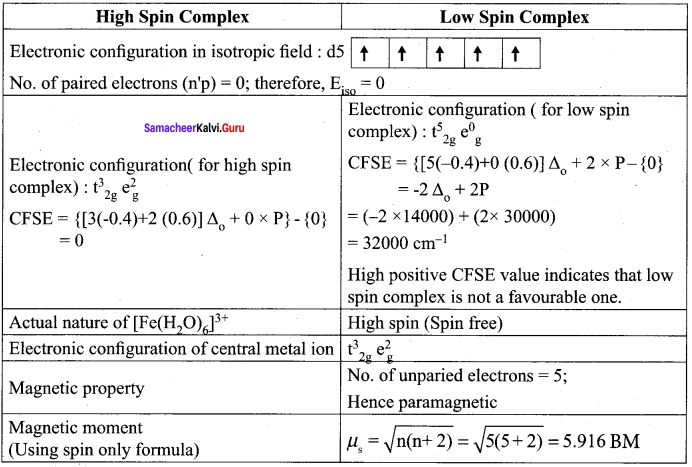
Question 12.
How would you measure CBSE for [Fe (CN)6]3-.
Answer:
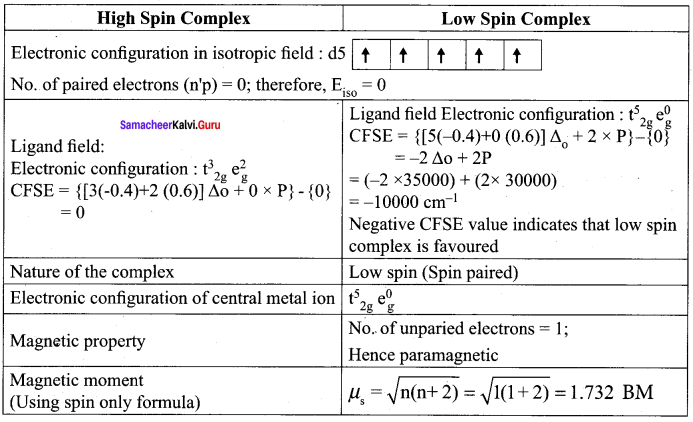
Question 13.
Explain about the classification of metal carbonyls with suitable examples. Classification:
Answer:
1. based on the number of metal atoms present. Depending upon the number of metal atoms present in a metalic carbonyl, they are classified as follows.
(a) Mono nuclear carbonyls. These compounds contain only one metal atom and have simple structures. For e.g., [Ni (CO)4] Nickel tetra carbonyl is tetrahedral, [Fe (CO)5] Iron pentacarbonyl is trigonalbipyramidal. [Cr (CO)6] Chromium hexacarbonyl is octahedral.
(b) Poly nuclear carbonyls Metallic carbonyls containing two or more metal atoms are called poly nuclear carbonyls. Poly nuclear metal carbonyls may be –
Homonuclear – ([CO2 (CO)6], [Mn2(CO)10], [Fe3(CO)12])
Fletero nuclear – ([MnCO (CO)9], [MnRe (CO)10])
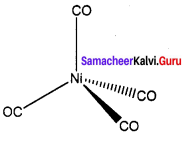
2. based on structure. The structures of binuclear metal carbonyls involve either metal – metal bonds or bridging Co groups or both. The carbonyl ligands that are attached to only one metal atom are referred to as terminal carbonyl groups, whereas those attached to two metal atoms simultaneously are called bridging carbonyls. Depending upon the structures of metal metal carbonyls they are classified as follows:
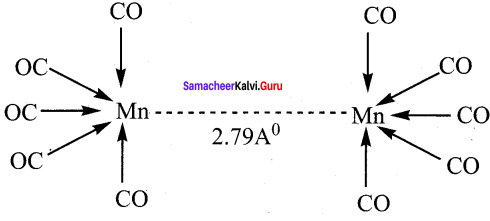
(a) Non – bridged metal carbonyls.
Example:- [Ni (CO)4]
These metal carbonyls do not contain any bridging carbonyl lieands. Thev mav be of two tvnes. Non- bridged metal carbonyls which contain terminal carbonyls as well as Metal- Metal bonds. For examples,The structure of Mn2(CO)10 . Contains one metal – metal bond, so the formula is more correctly represented as (CO)5Mn – Mn(CO)5.
(b) Bridged carbonyls:
These metal carbonyl contain one or more bridging carbonyl ligands along with terminal carbonyl ligands and one or more metal – metal bonds For Example: Fe (CO)9 Di-iron ennea carbonyl molecule consists of three CO ligands, six terminal CO groups and single Fe – Fe A bond formed by weak coupling of the unpaired electrons present in two 3d orbitals of 2 Fe atoms. The bond represented by dotted line is called fractional single bond.
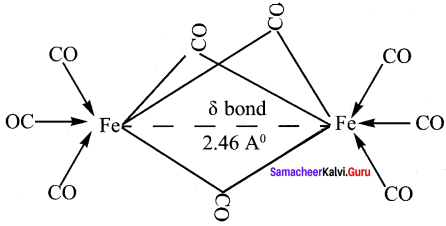
Question 14.
Explain about the importance and application of coordination complexes.
Answer:
1. Phthalo blue – a bright blue pigment is a complex of copper (II) ion and it is used in printing ink and packaging industry.
2. Purification of Nickel by Mond’s process involves formation of [Ni (CO)4] which yields 99.5% pure on decomposition.
3. EDTA is used as a chelating ligand for the separation of lanthanides, in softening of hard water and also in removing lead poisoning.
4. Coordination complexes are used in the extraction of silver and gold from their ores by forming soluble cyano complex. These cyano complexes are reduced by zinc to yield metals. This process is called Mac – Arthur Forrest cyanide process.
5. Some metal ions are estimated more accurately by complex formation. For eg., Ni2+ present in Nickel chloride solution is estimated accurately forming an insoluble complex called [Ni (DMG)2],
6. Many of the complexes are used as catalyst in organic and inorganic reactions. For e.g.,
- Wilkinson’s Catalyst – [(PPh3)3 Rh Cl] is used for hydrogenation of alkenes.
- Ziegler – Natta Catalyst [TiCl4 + Al (C2H5)3] is used in the polymerisation of ethene.
7. In photography, when the developed film is washed with sodium thio sulphate solution (hypo), the negative film gets fixed. Undecomposed AgBr forms a soluble complex called sodium dithio sulphate argentate (I) which can be removed easily by washing the film with water.
AgBr + 2Na2S2O3 → Na3 [Ag (S2O3)2] + 2NaBr
Question 15.
Write the formulae for the following coordination compounds:
Answer:
- Tetraamminediaquacobalt (III) chloride.
- Potassium tetracyanonickelate (II).
- Tris ( ethane – 1, 2 – diamine ) chromium (III) chloride.
- Amminebromidochloridonitrito – N – platinate (II).
- Dichlorobis(ethane – 1, 2 – diamine ) platinum (IV) nitrate.
- Iron (III) hexacyanoferrate (II)
Answer:
- [CO(NH3)4(H2O)2]Cl3
- K2[Ni(CN)4]
- [Cr(en)3]Cl3
- [Pt(NH3)BrCl(NO2)]–
- [PtCl2(en)2](NO3)2
- Fe4[Fe(CN)6]3
Question 16.
Write the IUPAC names of the following:
- [CO(NH3)6]Cl3
- [CO(NH3)5Cl]Cl2
- K3[Fe(CN)6]
- K3[Fe(C2O4)3]
- K2[PdCl4]
- [Pt(NH3)2Cl(NH2CH3)]Cl
Answer:
- Hexaamine cobalt (III) chloride
- Pentaamine chloridocobalt (III) chloride
- Potassium hexacyanoferrate (III)
- Potassium trioxalatoferrate (III)
- Potassium tetrachlorido palladate (II)
- Diamminechlorido (methylamine) platinum (II) chloride
Question 17.
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
- K[Cr(H2O)2(C2O4)2]
- [CO(NH)3Cl]Cl2
- K3[Fe(CN)6]
- K3[Fe(C2O4)3]
- K2[PdCl4]
- [Pt(NH3)2Cl(NH2CH3)]Cl
Answer:
1. Both geometrical (cis, trans) and optical isomer for cis – form.
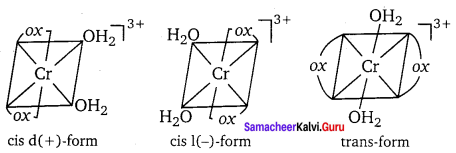
2. Two optical isomers can exist.
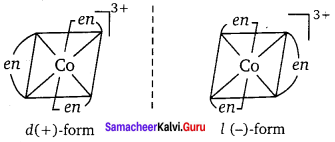
3. Linkage isomers [CO(NH3)5ONO](NO3)2 and [CO(NH3)5NO2](NO3)2
4. Geometrical isomerism.
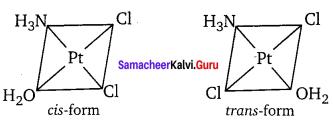
Question 18.
Give evidence that [CO(NH3)5Cl]SO4 and [CO(NH3)5SO4]Cl are ionisation isomers.
Answer:
When they are dissolved in water, they will give different ions in the solution which can be tested by adding AgNO3 solution and BaCl2 solution. When Cl ions are the counter ions, a white precipitate will be obtained with AgNO3 solution. If SO42- ions are the counter ions, a white precipitate will be obtained with BaCl2 solution.
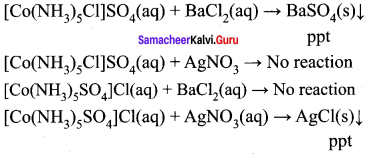
Question 19.
Explain on the basis of valence bond theory that [NI(CN)4]2- ion with square planar structure is diamagnetic and the [NI(CN)4]2- ion with tetrahedral geometry is paramagnetic.
Answer:
Nickel in [NI(CN)4]2- is in the +2 oxidation state, i.e. nickel is present as Ni2+ ion. dsp2 – hybrid orbitals
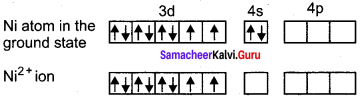
CN– is a strong ligand as it approaches the metal ion, So the electrons should getd paired up.

In [NI(CN)4]2-, Cl– provides a weak ligand field. It is therefore unable to pair up the unpaired electrons of the 3d orbital.
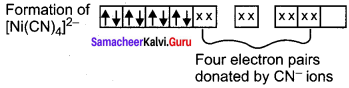
Hence the hybridisation is sp3, and it is para¬magnetic.
Question 20.
[NI(CN)4]2- is paramagnetic while [Ni(CO)4]3- is diamagnetic though both are tetrahedral. Why?
Answer:
In [NI(CN)4]2-, Ni is in +2 oxidation state with electronic configuration = 3d84s°.
![]()
Cl– is a weak ligand. It cannot pair up the electrons in 3d orbitals. Hence, it is paramagnetic. In [Ni(CO)4], Ni is in zero oxidation state and its configuration is -3d84S2. In the presence of CO ligand the 4s electrons shift to 3d orbital to pair up the 3d electrons. Thus, there is no unpaired electron present. Hence it is diamagnetic.
Question 21.
[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3- is weakly paramagnetic. Explain.
Answer:
In both the complexes, Fe is in +3 oxidation state with the configuration 3d5. CN– is a strong field ligand. In its presence, 3d electrons pair up leaving only one unpaired electron. The hybridisation is d2sp3 forming inner orbital complex. H2O is a weak ligand. In its presence 3d electrons do not pair up. The hybridisation is sp3d2 forming an outer orbital complex containing five unpaired electrons, hence it is strongly paramagnetic.
Question 22.
Explain [CO(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
Answer:
In [CO(NH3)6]3+, oxidation state of CO = +3 Electronic configuration = 3 d6
In presence of NH3, 3d electrons pair up leaving two d-orbitais empty. Hence, the hybridisation is d2p2 forming an inner orbital complex. In [Ni(NH3)6]2+.
Oxidation state = +2, Electronic Configuration = 3d8. In the presence of NH3, 3d electrons do not pair up. The hybridisation involved is , s3d2 and it forms an outer orbital complex.
Question 23.
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2- ion.
Answer:
Electronic Configuration of Pt = 5d96s1
![]()
For square planar shape, hybridisation = dsp2
Hence, the unpaired electron in 5d – orbital pair up to make one d-orbital empty for dsp2 hybridisation. Thus, there is no unpaired electron.
Question 24.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2-is diamagnetic. Explain why?
Answer:
Cr(24): [Ar] 4s13d5
Cr3+(24): [Ar] 4s° 3d3
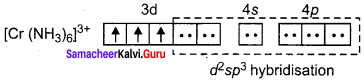
It is paramagnetic due to the presence of unpaired electrons.
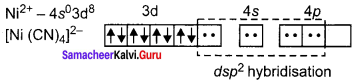
It has a square planar structure, and it is diamagnetic due to the absence of unpaired electrons.
Question 25.
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereo – chemistry and magnetic moment of the complex.
- K[Cr(H2O)2 (C2O4)2]. 3H2O
- [CO(NH3)5Cl]Cl2
- CrCl3(py)3
- Cs[FeCl4]
- K4[Min(CN)6]
Answer:
1. Potassium diaquabis (oxalato) chromate (III) trihydrate.
Cordination no. =6
Shape = Octahedral
Oxidation state of Cr = x + 0 + 2 (-2) = -1
(or)
x – 4 = – 1
(or)
x = + 3
Electronic configuration of Cr3+ = 3d3 = t2g 3eg0
No. of unpaired electrons (n) = 3
Magnetic moment (µ) = \(g\sqrt { n(n+2) } \) = \(g\sqrt { 3(5) } \) = \(g\sqrt { 15 } \) BM = 3.87BM
2. Pentaamminechloro cobalt (III) chloride Cordination no. of CO = 6
Shape = Octahedral Oxidation state of CO = x + 0 -1 = +2
(or)
x = +3
Electronic configuration of CO3+ = 3d6 = t2g6 eg0
n = 0
Magnetic moment (p) = 0
3. Trichlorotripyridine chromium (III), Cordination no, of Cr = 6
Shape = Octahedral Oxidation state of Cr =x – 3 + 0 = 0
x = +3
Electronic configuration of Cr3+ = 3d3 = t2g 3eg0
n = 3
Magnetic moment (µ) = \(g\sqrt { n(n+2) } \) = \(g\sqrt { 3(5) } \) = \(g\sqrt { 15 } \) BM = 3.87BM
4. Caesium tetrachloroferrate (III), Cordination no. of Fe =4
Shape = Tetrahedral Oxidation state of Fe = x – 4 = -1
x = + 3
Electronic configuration of Fe3+ = 3d5 = e2 t23
n = 5
(µ) = \(g\sqrt { 5(5+2) } \) = \(g\sqrt { 3(5) } \) = 5.92 BM
5. Potassium hexacyanomanganate (II), Cordination no. of Mn = 6
Shape = Octahedral Oxidation state of Mn = x – 6 = -4
x = +2
Electronic configuration of Mn2+ = 3d5 = t2g 5eg0
n =1
(µ) = \(g\sqrt { 1(1+2) } \) = \(g\sqrt { 3 } \) BM = 1.73 BM
Question 26.
How to find out stability constant by stepwise method?
Answer:
When a free metal ion is in aqueous medium, it is surrounded by (coordinated with) water molecules. It is represented as [MS6]. If ligands which are stronger than water are added to this metal salt solution, coordinated water molecules are replaced by strong ligands. Let us consider the formation of a metal complex ML6 in aqueous medium.(Charge on the metal ion is ignored) complex formation may occur in single step or step by step.
If ligands added to the metal ion in single step, then
[MS6]+6L \(\equiv\) [ML6]+6S
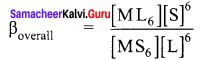
βoverall is called as overall stability constant. As solvent is present in large excess, its concentration in the above equation can be ignored.
∴ 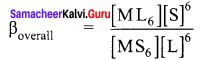
If these six ligands are added to the metal ion one by one, then the formation of complex [ML6] can be supposed to take place through six different steps as shown below. Generally step wise stability constants are represented by the symbol k.

Common Errors
- Complex salt and double salt- Students get confused
- Ligands name get confused
- IUPAC names may be difficult.
Rectifications
- Complex salt and double salt- Students get confused Complex salt have double brackets and double salt have no brackets.
- Ligands name get confused Neutral ligand names are different. Negative ligand names ends with letter ‘O’. Positive ligand names ends with ‘ium’.
- IUPAC names may be difficult. Cationic complex name starts with ligands name and Anionic complex names starts with first element whatever present.
We hope the Class 12th Samacheer Kalvi Chemistry Solutions Chapter 5 Coordination Chemistry Book Solutions Answers Guide Solutions provided here help the students to get the best knowledge. Also, students can use our Samacheer Kalvi Class 12th Chemistry Solutions Chapter 5 Coordination Chemistry Questions and Answers for better practice. You can contact us at any moment by giving comments in the comment section. Keep checking updates by bookmarking our website.