Students can Download Science Chapter 4 Chemistry in Daily Life Questions and Answers, Notes Pdf, Samacheer Kalvi 7th Science Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 7th Science Solutions Term 3 Chapter 4 Chemistry in Daily Life
Samacheer Kalvi 7th Science Chemistry in Daily Life Textual Evaluation
I. Choose the correct answers :
Question 1.
A drug effective in the treatment of pneumonia, and bronchitis, is ______
(a) Streptomycin
(b) Chloramphenicol
(c) Penicillin
(d) Sulphaguanidine
Answer:
(c) Penicillin
Question 2.
Aspirin is ______
(a) Antibiotic
(b) Antipyretic
(c) Sedative
(d) Psychedelic
Answer:
(b) Antipyretic
![]()
Question 3.
______ are that neutralize stomach acid.
(a) Antacid
(b) Antipyretic
(c) Analgesic
(d) Antihistanics
Answer:
(a) Antacid
Question 4.
The lowest temperature at which a substance catch the fire is called its ______
(a) Boiling point
(b) Melting point
(c) Critical temperature
(d) Ignition temperature
Answer:
(d) Ignition temperature
Question 5.
Which is the hottest part in the flame of candle ______
(a) Blue
(b) Yellow
(c) Black
(d) Way part
Answer:
(a) Blue
II. Fill in the blanks :
- Penicillin was first discovered by ______
- World ORS Day is ______
- Combustion is a chemical reaction in which and substance react with ______
- In the presence of water, the ignition temperature of paper is ______
- Fire produced by oil cannot be controlled by ______
Answer:
- Alexander Fleming
- July 29
- oxidizing agent
- not reached
- water
III. True or False – If False, give the correct answer :
Question 1.
Antibiotics does work for viruses like cold and
Answer:
(False)
Correct statement: Antibiotics does not work for viruses like cold and flu
Question 2.
Analgesics are the substances that lower the temperature during fever.
Answer:
(False)
Correct statement : Antipyretic are the substances that lower the temperature during fever.
![]()
Question 3.
All fuels form flame.
Answer:
(False)
Correct statement: All fuels do not form flame.
Question 4.
Oxygen is necessary for combustion.
Answer:
True
Question 5.
Burning wood and coal causes pollution of air.
Answer:
True
IV. Match the following :
Question 1.
| 1. Antipyretic | Reduce pain |
| 2. Analgesic | Reduce body temperature |
| 3. Antacid | Spontaneous combustion |
| 4. Phosphorus | ORS Solution |
| 5. Carbon – di – oxide | Leads to respiratory problem |
Answer:
| 1. Antipyretic | Reduce body temperature |
| 2. Analgesic | Reduce pain |
| 3. Antacid | ORS Solution |
| 4. Phosphorus | Spontaneous combustion |
| 5. Carbon – di – oxide | Leads to respiratory problem. |
V. Analogy:
Question 1.
Inner zone of flame:: ______, outer zone of flame : : ________
Anwer:
Black, Blue
Question 2.
Tincture:: ______, Histamine : : _______
Answer:
Antiseptic, Chemical messenger
VI. Very short answer :
Question 1.
First viral disease detected in human being was : _____ (Yellow fever / dengue fever)
Answer:
Yellow fever.
Question 2.
______,:_______,:_______ are called green house gases
Answer:
CO2 , Methane, Chlorofluorocarbons
Question 3.
Name a substance which can be used as an antiseptic as well as disinfectant.
Answer:
Garlic, Turmeric, Aloe vera.
![]()
Question 4.
What are the main constituents of dettol?
Answer:
Mixture of chloroxylenol and terpincol
Question 5.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
KJ/Kg.
Question 6.
How many types of combustion are there?
Answer:
- Rapid combustion
- Spontaneous combustion
- Explosion
Question 7.
What are the essential requirements for producing fire?
Answer:
Fuel, Heat and Oxygen.
VII. Short Answer Questions:
Question 1.
Why should not medicines be taken without consulting doctors?
Answer:
One should not take medicines without consulting doctors because if a wrong medicine is accidently eaten for a disease, it may not cure the disease but actually can have harmful side effects to the body.
Question 2.
Why do antiseptics differ from disinfectants? Give one example of each.
Answer:
Difference between Antiseptic and Disinfectants
| Antiseptic | Disinfectants | |
| 1. | All antiseptic are disinfectants
|
1. All disinfectants are not antiseptic
|
| 2. | It can be applied on the live tissue | 2. It can be apply on in animate object
|
| 3. | E.g. skin / Mucous | 3. E.g. Surface, lab working tables, floor.
|
Question 3.
What is ignition temperature?
Answer:
The minimum temperature at which a substance catches fire and burns is called its ignition temperature..
Question 4.
If 4.5kg of fuel is completely burnt and amount of heat produced stands measured at 1,80,000 KJ. What is the calorific value?
Answer:
Amount of fuel = 4.5 kg
Heat produced = 1,80,000 kj
Calorific value = ?
Solution:
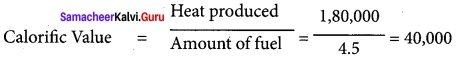
∴ Calorific value = 40,000 kj/kg
VIII. Answer in Detail:
Question 1.
Explain briefly about antibiotic and analgesic.
Answer:
- Many micro organisms and plants synthesize chemicals which are toxic in nature to protect them from invading organisms.
- Those biosynthesized chemicals can be isolated from the plants/micro organisms and was used as medicines against infectious diseases, these substances were called as antibiotics.
- Ex: Chloramphenicols, tetracyclines, Penicillin derivatives, cephalosporin’s and their derivatives.
- The world’s first antibiotic penicillin was discovered by Dr. Alexander Fleming.
Analgesics:
- Analgesics or pain killers that react like the pain-suppressing chemicals released by the body.
- They suppress the feeling of ‘pain.
- This analgesics drug selectively relieves pain by acting either in CNS (Central Nerves System) or on peripheral pain mechanism, without significantly altering consciousness.
Question 2.
Make labeled diagram of a candle flame.
Answer:
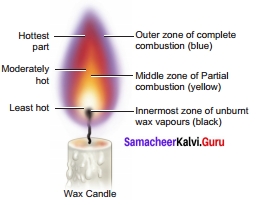
IX. Picture based question
Question 1.
Arul and Aakash were doing an experiment in which water was to be heated in a beaker. Arul kept the beaker near the wick in the yellow part of candle flame. Aakash kept the beaker in the outer most part to the flame. Whose water will get heated in a shorter time?
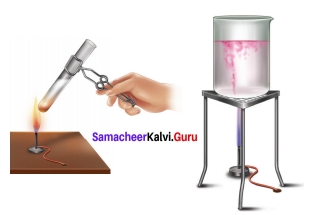
Answer:
The water heated by Akash will get heated in a shorter time because he kept his beaker near the hottest (non- luminous) zone of the flame. But Arul kept the beaker in the luminous zone which is moderately hot. So, it will take longer time.
Samacheer Kalvi 7th Science Chemistry in Daily Life Intext Activities
Activity
Question 1.
What happens when you add with these chemicals?
Sugar + Potassium permanganate + Glycerin

Answer:
- After adding sugar, potassium permanganate and glycerin to the dish, immediately step back because spark and solid potassium permanganate will be expelled from the dish.
- When potassium permanganate mixes with glycerin, a redox reaction starts. This reaction starts out really slow, but produces a lot of heat, so it will start to speed up bit by bit. As the potassium permanganate oxidises the sugar, it will speed up more and more until it finally starts to smoke and after that it will ignite.
Samacheer Kalvi 7th Science Chemistry in Daily Life Additional Questions
I. Choose the correct answer.
Question 1.
is a special combination of dry salt that is mixed with safe water.
(a) PRS
(b) ORS
(c) SOR
(d) ROS
Answer:
(b) ORS
Question 2.
Which of the following conditions is not necessary for combustion?
(a) Oxygen
(b) Adequate ignition temperature
(c) Combustible substance
(d) High calorific value
Answer:
(d) High calorific value
Question 3.
During diarrhoea the intestine is still able to absorb molecules.
(a) glucose
(b) sodium
(c) citrate
(d) none
Answer:
(a) glucose
![]()
Question 4.
Antacids are actually .
(a) weak acids
(b) weak bases
(c) strong acids
(d) strong bases
Answer:
(b) weak bases
Question 5.
Our stomach naturally produce to help digest and breakdown food.
(a) Sulphuric acid
(b) acetic acid
(c) hydrochloric acid
(d) none of these
Answer:
(c) hydrochloric acid
Question 6.
_______ are substances applied to the exterior of a body that kill microbes.
(a) Antiseptics
(b) Antihistamine
(c) Antipyretic
(d) ORS
Answer:
(a) Antiseptics
Question 7.
All combustion reactions are .
(a) endothermic
(b) exothermic
(c) both a and b
(d) None of these
Answer:
(b) exothermic
Question 8.
Any reaction that involves reaction with oxygen is called reaction.
(a) reduction
(b) ignition
(c) oxidation
(d) none
Answer:
(c) oxidation
![]()
Question 9.
Which is the luminous part of the plame?
(a) Outer zone
(b) Middle zone
(c) Inner zone
(d) None
Answer:
(b) middle zone
II. Fill in the Blanks.
- The chemical process in which a substance reacts with oxygen to produce heat is called _________
- Substances which vapourise during burning produce _________
- _________ is one that neutralize stomach acid
- _________ is a chemical messenger involved in number of complex biological reactions.
- _________ are used to treat the disease and to improve our health.
- Once infection is sensed, the immune system releases a chemical called _________
- Sensing the pyrogens, hypothalamus increases the body temperature by releasing a chemical _________
- _________ that react like the pain – suppressing chemicals released by the body.
- _________ resistane is defined as the ability of the microorganisms to resist the effects of an antibiotic to which they were once sensitive.
- The process of osmosis, the salts and sugars pull water into your bloodstream and speed up _________
Answer:
- Combustion
- flames
- Antacid
- Histamine
- Medicines
- pyrogen
- prostaglandin
- Analgesics
- Antibiotic
- rehydration
III. True or False – if false, give the correct statement.
Question 1.
If there is inadequate salt in the intestinal wall, the body will not be able to absorb water.
Answer:
True
Question 2.
Acidity issues arise when there is excess production of acetic acid due to triggers.
Answer:
Correct statement : Acidity issues arise when there is excess production of due to triggers.
Question 3.
The lining of our stomach with a pH of 4 to 6 is designed as such to withstand a high acidic environment.
Answer:
False
Correct statement: The lining of our stomach with a pH of 1 to 3 is designed as such to withstand a high acidic environment.
Question 4.
The bacteria staphylococcus is meant to cause deadly diseases such as pneumonia sour throat etc.
Answer:
True
Question 5.
Fleming named the mould penicillum notatum, from which the antibiotic penicillin was isolated.
Answer:
True
![]()
Question 6.
Paracetamol interact with the receptors and reduce the intensity of pain signals to the brain.
Answer:
True
Question 7.
Bacteria and virus can thrive above a certain temperature.
Answer:
Correct statement: Bacteria and virus thrive above a certain temperature.
Question 8.
The adverse effects of antihistamines are mouth dryness and sleepiness.
Answer:
True
Question 9.
Complete combustion of the fuel takes place and the colour of the flame is yellow and is the hottest part of the flame.
Answer:
False
Correct statement: Complete combustion of the fuel takes place and the colour of the flame is blue and is the hottest part of the flame.
IV. Match the following :
Question 1.
| 1. | White flame | (a) | Table salt |
| 2. | Indigo flame | (b) | Bleaching powder |
| 3. | Blue flame | (c) | Potassium chloride |
| 4. | Orange flame | (d) | Epsom salt |
Answer:
- d
- c
- b
- a
Question 2.
| 1. | Inner zone | (a) | Yellow |
| 2. | Outer zone | (b) | Borax powder |
| 3. | Middle zone | (c) | Blue |
| 4. | Green flame | (d) | Black |
Answer:
- d
- c
- a
- b
V. Very short Answers:
Question 1.
What is antipyretics?
Answer:
- Antipyretics (anti – against and pyretic – feverish) are chemical substances that reduce fever.
- They suppress the release of prostaglandin and reduce fever.
Question 2.
What is antiseptics?
Answer:
Antiseptics are substances applied to the exterior of a body that kill or inhibit microbes and infective agents.
Question 3.
Name few natural antiseptics.
Answer:
- Garlic
- Turmeric
- Aloevera.
Question 4.
Define histamine.
Answer:
Histamine is a chemical messenger involved in number of complex biological reactions.
Question 5.
Complete the oxidation reaction.
Answer:
CH4 + 2O2 → ______ + ______ + Heat energy
CO2 + 2H2O
Question 6.
Name a few substances which produce flames on burning.
Answer:
Wax, Kerosene.
Question 7.
Give one example for spontaneous combustion.
Answer:
Phosphorous burns spontaneously at room temperature.
Question 8.
Give one example for slow combustion.
Answer:
Respiration.
![]()
Question 9.
What are the classes of fire?
Answer:
There are 5 classes of fire.
- Class A
- Class B
- Class C
- Class D
- Class D.
Question 10.
Give example for class B fires.
Answer:
Petrol, turpentine or paint.
VI. Short Answer.
Question 1.
Mention some of the common antacids.
Answer:
- Sodium bicarbonate (NaHC03)
- Calcium carbonate (CaC03)
- Magnesium Hydroxide (MgOH)2
- Magnesium carbonate (MgCOs)
- Aluminium Hydroxide Al (OH)3
Question 2.
What is antibiotic resistance?
Answer:
Antibiotic resistance is defined as the ability of the microorganisms to resist the effects of an antibiotic to which they were once sensitive.
![]()
Question 3.
What is fever?
Answer:
In normal course, our body temperature is one degree below or one degree above 98.6 degrees Fahrenheit. When the temperature goes above this level it is called fever.
Question 4.
Mention the ways to intake the medicine.
Answer:
- Oral use
- External use
- Injections (Intramuscular/Intra venous).
Question 5.
What do you mean by inflammable substance?
Answer:
- Substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances.
- E.g. Petrol. Alcohol, LPG (Liquefied Petroleum Gas), CNG (Compressed Natural Gas), etc.
Question 6.
Write a note on flame.
Answer:
- Flame is actually a chemical reaction.
- To be specific, the flame is a mixture of gases (vaporized fuel, oxygen, carbon dioxide, carbon monoxide, water vapor, and many volatile materials) and so is matter.
- The light and heat produced by the flame is energy, not matter. But fire is a matter.
Question 7.
What is slow combustion? Give example.
Answer:
Slow combustion is a form of combustion which takes place at low temperatures. Respiration is an example of slow combustion.
Question 8.
Mention the conditions necessary for producing fire.
Answer:
The conditions necessary for producing fire are,
- Fuel
- Air (to supply oxygen)
- Heat (to raise the temperature of the fuel beyond its ignition temperature)
Question 9.
Mention the most common types of fire extinguishers.
Answer:
- Air pressurized water extinguishers
- Carbon-di-oxide extinguishers
- Dry chemical powder extinguishers.
VII. Long Answer
Question 1.
Explain the structure of a candle flame.
Answer:
A candle flame has three main zones, they are
- The outer zone – complete combustion of the fuel takes place and the colour of the flame is blue and is the hottest part of the flame. It is the non-luminous part of the flame.
- The middle zone – partial combustions of the fuel takes place and the colour of the flame is yellow and is moderately hot part of the flame. It is the luminous part of the flame.
- The inner zone – There are unburnt vapours of the fuel and the colour is black and is least hot part.
Question 2.
Write the characteristics of a good fuel.
Answer:
- Readily available
- Cheap
- Easy transport and store
- Burns at moderate rate
- Produce large amount of heat
- Do not leave behind any undesirable substances.
- Does not cause pollution.
![]()
Question 3.
How do fire extinguishers work? Explain.
Answer:
- Portable fire extinguishers apply an extinguishing agent that will either cool burning fuel, displace or remove oxygen, or stop the chemical reaction so fire cannot continue to burn.
- When the candle of an extinguisher is compressed, it opens and inner canister of high pressure gases forces the extinguishing agent from the main cylinder through a siphon tube and out the nozzle.
- A fire extinguisher works much like a can of hair spray.
VIII. HOTS:
Question 1.
Why a person is covered with a blanket when the clothes of that person catch fire?
Answer:
When the clothes of a person catch fire, the person is covered with a blanket to extinguish
the fire. This is because the supply of air to the burning clothes is cut off by blanket, due to this the clothes stop burning and the fire gets extinguished.
![]()
Question 2.
Why is the candle flame straight?
Answer:
A candle flame is caused by vapour burning above the candle. This burning vapour is hotter than the surrounding air and is therefore less dense. So, by the principle of convection, it “rises” so the flame is always upwards