Students can Download Science Term 3 Chapter 5 Acids and Bases Questions and Answers, Notes Pdf, Samacheer Kalvi 8th Science Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 8th Science Solutions Term 3 Chapter 5 Acids and Bases
Samacheer Kalvi 8th Science Acids and Bases Text Book Exercise
I. Choose the best answer:
Question 1.
Acids are ……………. in taste.
(a) sour
(b) sweet
(c) bitter
(d) salty
Answer:
(a) sour
Question 2.
Aqueous solutions of ……………. conduct electricity.
(a) acid
(b) base
(c) salt
(d) salt and base
Answer:
(d) salt and base
![]()
Question 3.
In acidic solution, blue litmus changes into ……………. colour.
(a) blue
(b) green
(c) red
(d) white
Answer:
(c) red
Question 4.
Base is a substance that gives ……………. on dissolving in water.
(a) OH–
(b) H+
(c) OH
(d) H
Answer:
(a) OH
![]()
Question 5.
Sodium hydroxide is a …………….
(a) acid
(b) base
(c) oxide
(d) alkali
Answer:
(d) alkali
Question 6.
Red ant sting contains …………….
(a) acetic acid
(b sulphuric acid
(c) oxalic acid
(d) formic acid
Answer:
(d) formic acid
Question 7.
Magnesium oxides are used for treating …………….
(a) acidity
(b) head pain
(c) teeth decay
(d) None of these
Answer:
(a) acidity
![]()
Question 8.
Acid mixed with base forms …………….
(a) salt and water
(b) salt
(c) water
(d) no reaction
Answer:
(a) salt and water
Question 9.
We brush our teeth with tooth paste because it is …………….
(a) basic
(b) acidic
(c) both a and b
(d) none of these
Answer:
(a) basic
Question 10.
In basic solution turmeric indicator paper changes from yellow to …………….
(a) blue
(b) green
(c) yellow
(d) red
Answer:
(d) red
![]()
II. Fill in the blanks:
- Benzoic acids are used for …………
- The word sour refers to ………… in Latin.
- Bases are ………… in taste.
- Chemical formulae of calcium oxide is …………
- Wasp sting contains …………
- Turmeric is used as a …………
- In acidic solution, the colour of the hibiscus indicator paper will change to …………
Answer:
- preservation of food
- acidus
- bitter
- CaO
- alkaline substance
- indicator
- deep pink or deep red
![]()
III. State True or False. If false, correct the statement:
Question 1.
Most of the acids are not soluble in water.
Answer:
True.
Question 2.
Acids are bitter in taste.
Answer:
False
Correct statement:
Acids are sour in taste. Bases are bitter in taste.
Question 3.
Bases are soapy to touch when they are dry.
Answer:
False
Correct statement:
Bases are soapy to touch only in aqueous media, not in dry nature.
Question 4.
Acids are corrosive in nature.
Answer:
True
![]()
Question 5.
All bases are alkalis.
Answer:
False
Correct statement:
All alkalis are base, but all bases are not alkalis.
Question 6.
Hibiscus flower is an example for natural indicator.
Answer:
True
![]()
IV. Answer briefly:
Question 1.
Define acid.
Answer:
A substance which contains one or more replaceable hydrogen atoms.
Question 2.
Write any four physical properties of acids.
Answer:
- Acids are sour in taste.
- They are corrosive in nature. Strong acids can spoil substances like human skin, clothes and paper.
- Generally acids exist in liquid state but few acids exist in solid state too. E.g. Benzoic acid.
- Acids are colourless.
- Acids change the colour of the indicators. Blue litmus paper turns red and methyl orange turns pink when treated with acids.
![]()
Question 3.
What are the similarities between acids and bases?
Answer:
- They are corrosive in nature.
- They undergo ionization in aqueous solution.
- They conduct electricity in aqueous solution.
- They undergo neutralization reaction.
Question 4.
State the difference between acids and bases.
Answer:
Difference between acids and bases:
Acids:
- They produce H+ ions in water.
- They are sour in taste.
- Few acids are in solid state.
- Acids turn blue litmus paper red.
Bases:
- They produce OH– ions in water.
- They are bitter in taste.
- Most of the bases are in solid state.
- Bases turn red litmus paper blue.
![]()
Question 5.
What is an indicator?
Answer:
An indicator or acid – base indicator is a chemical substance which indicates the acidic or basic nature of a solution by suitable colour change.
Question 6.
What is a neutralization reaction?
Answer:
Neutralization is a chemical reaction in which an acid and a base react with each other to form water and salt.
Question 7.
Write any four physical properties of base.
Answer:
- Bases generally exist in solid state but some bases exist in liquid state also. E.g. Ammonium hydroxide, calcium hydroxide.
- Bases give soapy touch only in aqueous media not in dry nature.
- Bases are bitter in taste.
- Bases are corrosive in nature. When come in contact with the skin frequently they form painful blisters.
- Bases also change the colour of the indicators. Red litmus paper turns blue when . treated with bases. Similarly, they turn methyl orange yellow and phenolphthalein pink.
![]()
V. Answer in detail:
Question 1.
What are the uses of acids?
Answer:
- Hydrochloric acid present in our stomach helps in the digestion of foodstuff.
- Vinegar (acetic acid) is used to preserve food materials.
- Benzoic acid is also used to preserve food materials like pickles.
- Sodium or potassium salts of higher fatty acids are used to make washing and bathing soaps.
- Sulphuric acid is called the king of chemicals. It is an effective dehydrating agent. It is used in various industries to make detergents, paints, fertilizers and many more chemicals.
- Hydrochloric acid, Nitric acid and Sulphuric acid are important laboratory reagents.
- Cells of all living organisms contain the fundamental nuclear material called nucleic acids. Animals have deoxyribo nucleic acid (DNA) whereas plants contain ribo nucleic acid (RNA).
Question 2.
What are the uses of bases?
Answer:
- Potassium hydroxide is used to make bathing soaps.
- Sodium hydroxide is used to make washing soaps.
- Sodium hydroxide is also used in paper industries, textile industries and in the preparation of medicines.
- Calcium hydroxide is used for white washing.
- Aluminum hydroxide and magnesium hydroxides are used in antacids to cure acidity problems.
- Ammonium hydroxide is used to manufacture fertilizers, nylon, plastics and rubber.
![]()
Question 3.
Explain the neutralization reaction in our daily life.
Answer:
1. Ant bite:
- Whenever bees or red ants bite they inject an acid called formic acid.
- These acids cause burning sensation and pain.
- To suppress the pain, a suitable base in the form of calcium hydroxide (readily available at home) is applied to neutralise the formic acid.
2. Wasp bite:
- When we are bitten by wasp, we feel the burning sensation and pain. It is due to an alkaline substance injected by the insect.
- To neutralise the alkalinity, we use vinegar which is an acid.
3. Tooth decay:
- The bacteria present in our mouth decompose the food particles stuck in the gaps between our teeth thereby causing acid formation which leads to tooth decay.
- When we brush with tooth powder or tooth paste containing weak bases, the acid gets neutralized.
4. Acidity:
- Excessive production of hydrochloric acid in our stomach causes ulcer in stomach and food pipe.
- In order to neutralize, antacids which are nothing but weak bases like aluminum and magnesium hydroxides are used.
5. Agriculture:
Farmers add lime fertilisers such as powdered lime (CaO), limestone (CaCO3) or ashes of
burnt wood to the soil to neutralise the acidity.
6. Industries:
Effluents from the industries contain acids such as sulphuric acid. It is treated by adding lime to neutralise it before it is discharged into rivers and streams.
![]()
Question 4.
How will you prepare natural indicator from turmeric powder?
Answer:
- Turmeric indicator is one of the natural indicator.
- By adding small amount of water to turmeric powder, a paste is prepared.
- This is applied on a blotting paper or filter paper and dried.
- These strips are used as indicators to find the nature of the solution.
- In acidic solution, turmeric indicator paper has no change in colour.
- That means, it remains yellow. In basic solution, the colour changes from yellow to red.
VI. Higher Order Thinking Questions:
Question 1.
Vinu and Priyan take their lunch at school. Vinu eats lemon rice and Priyan eats curd rice. Both lemon rice and curd rice are sour in taste. What is the reason?
Answer:
- Curd contains lactic acid. The lactic acid makes curd rice sour in taste.
- Lemon juice contains citric acid. The citric acid makes lemon rice sour in taste.
- Generally acids are sour in taste.
![]()
Question 2.
Heshna and Keerthi are friends. Keerthi’s teeth are white without caries, but Heshna has teeth with caries. Why? How is it formed?
Answer:
- Caries is caused by the action of acids on the enamel surface.
- The acid is produced when sugar in foods or drinks react with bacteria present on the tooth surface.
- Heshna has not cleaned her teeth well after sipping sugary drinks and snacking.
- She has to brush after meals and before bed.
Samacheer Kalvi 8th Science Acids and Bases Additional Questions
I. Choose the correct answer:
Question 1.
Acids present in fruits and vegetables are called ……………… acids.
(a) organic
(b) strong
(c) mineral
(d) fruit
Answer:
(a) organic
Question 2.
Vinegar is ……………… in taste.
(a) bitter
(b) sour
(c) sweet
(d) sweetless
Answer:
(b) sour
Question 3.
Citric acid is present in ………………
(a) curd
(b) milk
(c) lemon
(d) spinach
Answer:
(c) lemon
Question 4.
Which of the following is not a natural indicator?
(a) Litmus
(d) Turmeric
(c) Methyl orange
(d) Hibiscus
Answer:
(c) Methyl orange
![]()
Question 5.
An acid is ………………
(a) bitter is taste
(d) soapy to touch
(c) corrosive in nature
(d) all the above
Answer:
(c) corrosive in nature
![]()
Question 6.
The common salt is ………………
(a) sodium carbonate
(b) sodium bicarbonate
(c) sodium nitrate
(d) sodium chloride
Answer:
(d) sodium chloride
Question 7.
Add few drops of hibiscus indicator in soap solution. What do you observe?
(a) It turns green
(b) It turns magenta
(c) It turns yellow
(d) It turns red
Answer:
(a) It turns green
Question 8.
Acids and bases can be identified in the laboratory by ………………
(a) an indicator
(b) tasting
(c) touching
(d) smelling
Answer:
(a) an indicator
![]()
Question 9.
Lemon juice will turn ……………….
(a) phenolphthalein pink
(b) red litmus blue
(c) turmeric indicator red
(d) methyl orange red
Answer:
(d) methyl orange red
Question 10.
A salt may be ………………
(a) acidic only
(b) basic only
(c) neutral only
(d) acidic, basic or neutral
Answer:
(d) acidic, basic or neutral
![]()
II. Fill in the blanks:
- Acids are ……………… in taste.
- Solutions of acids conduct ………………
- ……………… change the colour of the indicators
- ……………… is used to preserve food materials
- ……………… is called the king of chemicals.
- Bases are chemical substances that are corrosive and ……………… in nature
- Water soluble bases are called ………………
- Sodium carbonate is commercially called as ………………
- Acidity or indigestion in stomach is due to excessive secretion of ………………
- Methyl orange gives ……………… colour in an acidic solution.
Answer:
- sour
- electricity
- Acids
- Vinegar
- Sulphuric acid
- bitter
- alkalis
- Baking soda
- hydrochloric acid
- majenta
![]()
III. Match the following:
Question 1.
- Wasp sting – (a) Milk of magnesia
- Common salt – (b) Acetic acid
- Organic acid – (c) Sodium chloride
- Antacid – (d) Vinegar
Answer:
- d
- c
- b
- a
![]()
Question 2.
- Sulphuric acid – (a) Weak base
- Sodium hydroxide – (b) Strong acid
- Acetic acid – (c) Strong base
- Ammonium hydroxide – (d) Weak acid
Answer:
- b
- c
- d
- a
Question 3.
- Sodium chloride – (a) Detergents
- Sodium carbonate – (b) Purification of water
- Sodium bicarbonate – (c) Taste to food
- Potash alum – (d) Antacids
Answer:
- c
- a
- d
- b
IV. True or False – if false, give the correct statement:
Question 1.
Acids have a sour taste and they are soapy to touch.
Answer:
False
Correct statement:
Bases have a sour taste and they are soapy to touch.
![]()
Question 2.
Neutral substances do not bring about any change in colour of indicators.
Answer:
True
Question 3.
All bases are alkalis but all alkalis are not bases.
Answer:
False
Correct statement:
All alkalis are bases but all bases are not alkalis.
![]()
Question 4.
When an acid reacts with a base, neutralisation reaction takes place to give salt and water.
Answer:
True
Question 5.
Acids are corrosive in nature. They corrode metals hence are not stored in metal containers.
Answer:
True.
![]()
V. Assertion and Reason:
Mark the correct choice as:
(a) If both assertion and reason are true and the reason is the correct explanation of assertion.
(b) If both assertion and reason are true, but reason is not the correct explanation of assertion.
(c) If the assertion is true, but reason is false.
(d) If the assertion is false, but reason is true.
(e) If both assertion and reason are false.
Question 1.
Assertion: To neutralise the excess acid formed in the stomach, milk of magnesia is taken.
Reason: Milk of magnesia contains a base called magnesium hydroxide.
Answer:
(a) Both assertion and reason are true and the reason is the correct explanation of assertion.
Question 2.
Assertion: A salt is produced when an acid is neutralised by a base.
Reason: A salt can be acidic, basic or neutral.
Answer:
(c) Both assertion and reason are true, but reason is not the correct explanation of assertion.
![]()
Question 3.
Assertion: With some samples of acids and bases, turmeric paper turns red.
Reason: Such samples are acidic in nature.
Answer:
(c) Assertion is true, but reason is false.
Correct Reason:
Such samples are basic in natural.
![]()
Question 4.
Assertion: Methyl orange and phenolphthalein are natural indicators.
Reason: Methyl orange is yellow in colour while phenolphthalein is pink.
Answer:
(d) Both assertion and reason are false.
VI. Very short answer questions:
Question 1.
Write the chemical name and formula of baking soda.
Answer:
Chemical name:
Sodium hydrogencarbonate
Formula: NaHCO3.
![]()
Question 2.
What is the colour of phenolphthalein in basic solution?
Answer:
Phenolphthalein is pink in basic solution.
Question 3.
Give names of any two natural indicators.
Answer:
Turmeric and Litmus.
Question 4.
Name the acid present in vinegar.
Answer:
Acetic acid.
![]()
Question 5.
Name any two synthetic acid-base indicators.
Answer:
Methyl orange, Phenolphthalein.
Question 6.
Which acid is present in milk?
Answer:
Lactic acid.
![]()
Question 7.
What is the reaction between an acid and a base called?
Answer:
Neutralisation reaction.
Question 8.
Name the base which is present in a window cleaner.
Answer:
Ammonium hydroxide.
![]()
Question 9.
Which chemical is used to neutralise the acidic soil?
Answer:
Quicklime.
Question 10.
Can we taste acids and bases to identify them?
Answer:
No, acids and bases are corrosive in nature.
![]()
VII. Short Answer Questions:
Question 1.
Are all acids corrosive in nature? Name few acids which are non-corrosive and may be part of our food.
Answer:
No, all acids are not corrosive in nature. Certain acids like acetic acid present in vinegar, citric acid present in citrus fruits, lactic acid present in curd, oxalic acid present in tomatoes, etc, are part of our food and non-corrosive in nature.
Question 2.
Write the important uses of hydrochloric acid and sulphuric acids.
Answer:
- Uses of hydrochloric acid – Cleaning agent for toilets, important laboratory agents.
- Uses of sulphuric acid –
- Used in car batteries.
- Used to prepare a large number of compounds.
Question 3.
How will you prepare china rose indicator?
Answer:
- Collect some china rose petals and place them in a beaker.
- Add some warm water, keep the mixture for some time till water becomes coloured.
- This coloured water is used as an indicator.
![]()
Question 4.
Give an important use of neutralisation reaction in daily life.
Answer:
- In our daily life, neutralisation reaction is used to cure indigestion.
- Too much acid in the stomach causes indigestion.
- An antacid such as milk of magnesia is used to neutralise the excessive acid.
Question 5.
What is the role of toothpaste in prevention of tooth decay?
Answer:
When we brush with tooth powder or tooth paste containing weak bases, the acid gets neutralized. So our teeth will be strong and healthy and can be protected from caries.
![]()
Question 6.
How to treat effluents from the industries?
Answer:
- Effluents from the industries contain acids such as sulphuric acid.
- It is treated by adding lime to neutralise it before it is discharged into rivers and streams.
Question 7.
Write the chemical equation for the reaction of bases with metal oxides.
Answer:
- All bases react with non-metallic oxides to form salt and water.
- Eg: Sodium hydroxide reacts with carbon dioxide to form sodium carbonate.
- Sodium hydroxide + Carbon dioxide —> Sodium carbonate + Water
NaOH + CO —> Na2CO3 + H2O
Question 8.
Write the reaction between sulphuric acid and water.
Answer:
Sulphuric acid + Water → Hydrogen ion + Sulphate ion
H2SO4 + H2O → 2H+ + \({ SO }_{ 4 }^{ 2- }\)
Question 9.
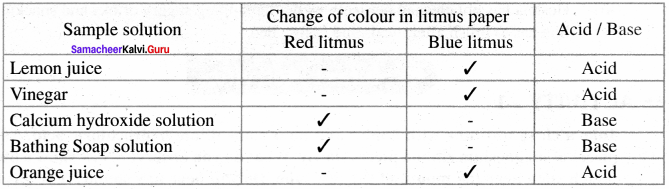
Complete the table

Answer:

VIII. Long answer questions:
Question 1.
Write a note on:
- Ant bite
- Wasp bite.
Answer:
1. Ant bite:
- Whenever bees or red ants bite they inject an acid called formic acid.
- These acids cause burning sensation and pain.
- To suppress the pain, a suitable base in the form of calcium hydroxide (readily available at home) is applied to neutralise the formic acid.
2. Wasp bite:
- When we are bitten by wasp, we feel the burning sensation and pain.
- It is due to an alkaline substance injected by the insect.
- To neutralize the alkalinity we use vinegar which is an acid to neutralise.
![]()
Question 2.
Write a note on:
- Litmus
- Phenolphthalein
- Methyl orange
Answer:
1. Litmus:
- Litmus is the most common indicators used in the laboratories.
- Litmus is a natural indicator which is extracted from lichens.
- It is available in the form of solution or in the form of strips prepared by absorbing litmus solution on filter paper.
- It is either red or blue in colour.
- Blue litmus paper turns red in acidic solution and red litmus paper turns blue in the basic solution.
2. Phenolphthalein:
- Phenolphthalein is a colourless compound.
- Its alcoholic solution is used as an indicator.
- It is colourless in acidic solution but turns pink in basic solution.
3. Methyl orange:
- Solid methyl orange dissolved in hot water and its filtrate is used as an indicator.
- It turns red in acidic solution and yellow in basic solution.
![]()
IX. Higher Order Thinking Questions:
Question 1.
What is the difference between an ant sting and a wasp sting?
Answer:
1. Ant sting:
An ant sting is acidic as it contains formic acid while the wasp sting is basic in nature.
2. Wasp sting:
Ant sting can be neutralised by a base like baking soda while wasp sting is neutralised by an acid like vinegar (acetic acid).
Question 2.
Copper or brass cooking vessels are coated with tin metal. Why?
Answer:
- Copper or brass cooking vessels are coated with tin metal (eyam).
- If it is not coated, the organic acids present in the food materials will react with copper and make the food poisonous.
- The tin isolates the vessel from the action of acids and prevents food poisoning.
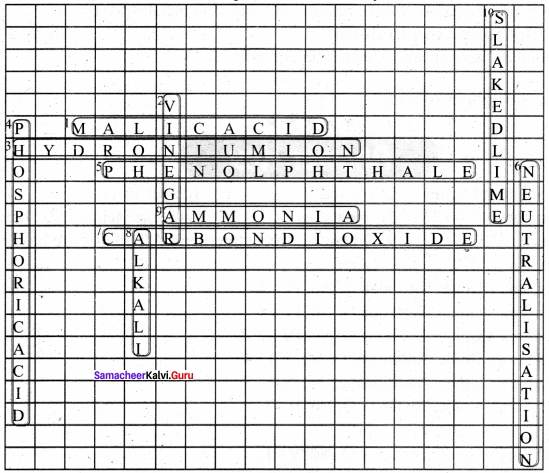
X. Solve the crossword by using the clues that below:
Across:
1. The acid found in apples.
3. The ion formed when an acid is dissolved in water.
5. An indicator that remains colourless in an acid.
7. The gas liberated when dilute hydrochloric acid reacts with limestone.
9. A gas that dissolved in water to form ammonium hydroxide.
Down:
2. An acid used for cooking.
4. An acid that has three hydrogen atoms in its moelcule.
6. A reaction in which an acid reacts w’ith a base to form salt and water only.
8. A base that is soluble in water.
10. The chemical name of this compound is calcium hydroxide.
Answer:
Samacheer Kalvi 8th Science Acids and Bases Intext Activities
Activity – 1
Question 1.
Take a clean test tube with holder and pour some dilute hydrochloric acid. Add few pieces of magnesium ribbon pieces slowly. What do you observe? Now show a burning match stick near the mouth of the test tube. Do you hear any sound? The gas burns with a pop sound. From this it is observed that hydrogen gas has been formed due to the reaction between acid and metal.
Answer:
The gas bums with a pop sound. From this it is observed that hydrogen gas has been formed due to the reaction between acid and metal.
![]()
Activity – 2
Take some lemon juice in a tumbler and add baking soda slowly. What do you see? I What do you infer from this?
Answer:
Inference:
When lemon juice is mixed with baking soda, the new product CO2 is formed with water and salt.
Activity- 3
Classify the following substances. Sodium oxide, Potassium hydroxide, Calcium oxide, Copper oxide, Calcium hydroxide, Ammonium hydroxide, Ferric hydroxide, Zinc oxide
Answer:

Activity – 4
Question 1.
Take a shirt with turmeric powder strain. Wash the shirt with soap. Do you observe any change in the colour? Why?
Answer:
Yes, the colour changes from yellow to red, because soapy solution is a base.
![]()
Activity – 5
Question 1.
Take a small beet root vegetable and cut it into pieces. Boil them in hot water and filter the extract. Take two test tubes. Take sodium hydroxide solution in one test tube and vinegar or lemon juice in another test tube. Add beet root extract slowly. Observe the colour change. What do you infer?
Answer:
- Observation – When beetroot juice is added with sodium hydroxide solution it turns into greenish yellow shows that NaOH– is a base.
- When it is added with lemon juice, the colour of beetroot juice remains same shows that lemon juice is acidic.
Activity – 6
Find out the nature of the solution